Transl Clin Pharmacol.
2019 Jun;27(2):64-68. 10.12793/tcp.2019.27.2.64.
Antiepileptic drug-induced severe cutaneous adverse reactions and HLA alleles: A report of five cases with lymphocyte activation test
- Affiliations
-
- 1Department of Clinical Pharmacology, Inje University College of Medicine, Busan Paik Hospital, Busan 47392, Republic of Korea. phshinjg@gmail.com
- 2Center for Personalized Precision Medicine of Tuberculosis, Inje University College of Medicine, Busan 47392, Republic of Korea.
- 3Department of Internal Medicine, Inje University College of Medicine, Busan Paik Hospital, Busan 47392, Republic of Korea.
- 4Department of Internal Medicine, Inje University College of Medicine, Haeundae Paik Hospital, Busan 48108, Republic of Korea.
- 5Department of Microbiology and Immunology, Inje University College of Medicine, Busan 47392, Republic of Korea.
- 6Department of Pharmacology and PharmacoGenomics Research Center, Inje University College of Medicine, Busan 47392, Republic of Korea.
- KMID: 2457573
- DOI: http://doi.org/10.12793/tcp.2019.27.2.64
Abstract
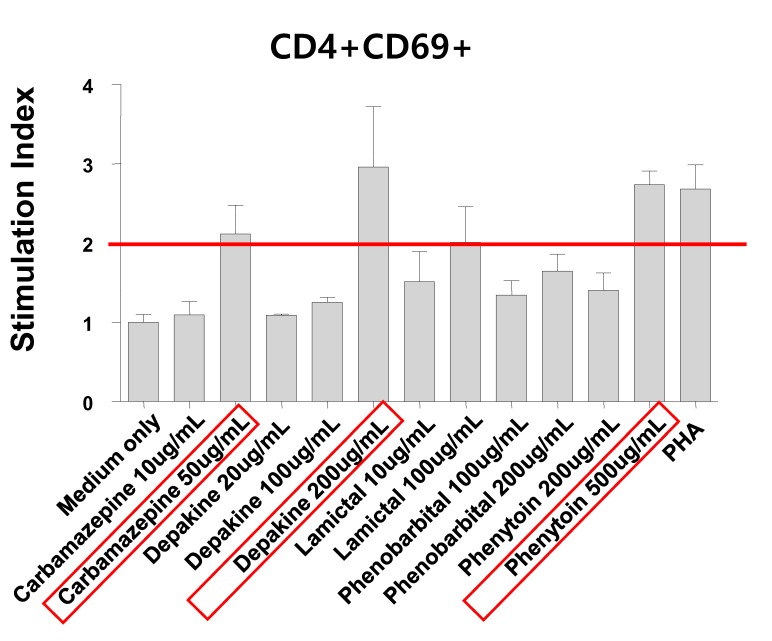
- Antiepileptic drugs (AEDs) can induce severe cutaneous adverse reactions (SCARs) such as Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome. We performed HLA genotyping and lymphocyte activation tests (LATs) for five AED-induced SCAR patients (three males and two females; aged 40-66 years old). Three patients were treated with carbamazepine (CBZ) for pain control, one was treated with phenytoin (PHT) for seizure prevention, and one was treated with valproic acid (VPA) for seizure prevention. One patient was diagnosed with CBZ-induced DRESS syndrome and the remaining patients were diagnosed with SJS. All patients recovered from SCARs after stopping suspicious drugs and supportive care. LATs were conducted to confirm the culprit drug responsible for inducing SCARs; and LAT results were positive for the suspected culprit drugs, in all except in one case. HLA-A, -B, and -C alleles were determined using PCR-sequence-based typing method. The common alleles of HLA were -A*02:01, -B*51:01, and -C*03:04 which were carried by three patients (60%) for each allele. The patient with CBZ-induced DRESS syndrome carried the HLA-A* 31:01 allele. One patient with CBZ-induced SJS and one patient with VPA-induced SJS carried the HLA-B*15:11 allele. No patients carried the HLA-B*15:02 allele, which is a known risk allele of AED-induced SCARs. Further investigation of the three common alleles found in the five AED-induced SCARs patients is needed. We demonstrated the usefulness of LAT for confirming the culprit drug.
Keyword
MeSH Terms
-
Alleles*
Anticonvulsants
Carbamazepine
Cicatrix
Drug Hypersensitivity Syndrome
Female
HLA-A Antigens
Humans
Long-Acting Thyroid Stimulator
Lymphocyte Activation*
Lymphocytes*
Male
Methods
Phenytoin
Seizures
Stevens-Johnson Syndrome
Valproic Acid
Anticonvulsants
Carbamazepine
HLA-A Antigens
Long-Acting Thyroid Stimulator
Phenytoin
Valproic Acid
Figure
Reference
-
1. Knowles SR, Shapiro LE, Shear NH. Anticonvulsant hypersensitivity syndrome: Incidence, prevention and management. Drug Saf. 1999; 21:489–501. PMID: 10612272.2. Blaszczyk B, Lasoń W, Czuczwar SJ. Antiepileptic drugs and adverse skin reactions: An update. Pharmacol Rep. 2015; 67:426–434. DOI: 10.1016/j.pharep.2014.11.009.PMID: 25933949.3. Roujeau JC. Immune mechanisms in drug allergy. Allergol Int. 2006; 55:27–33. PMID: 17075283.
Article4. Chung WH, Hung SI, Hong HS, Hsih MS, Yang LC, Ho HC, et al. Medical genetics: A marker for Stevens-Johnson syndrome. Nature. 2004; 428:486. PMID: 15057820.5. Ozeki T, Mushiroda T, Yowang A, Takahashi A, Kubo M, Shirakata Y, et al. Genome-wide association study identifies HLA-A*3101 allele as a genetic risk factor for carbamazepine-induced cutaneous adverse drug reactions in Japanese population. Hum Mol Genet. 2011; 20:1034–1041. DOI: 10.1093/hmg/ddq537. PMID: 21149285.
Article6. Ramírez E, Bellón T, Tong HY, Borobia AM, de Abajo FJ, Lerma V, et al. Significant HLA class I type associations with aromatic antiepileptic drug (AED)-induced SJS/TEN are different from those found for the same AED-induced DRESS in the Spanish population. Pharmacol Res. 2017; 115:168–178. DOI: 10.1016/j.phrs.2016.11.027. PMID: 27888155.
Article7. Chang CC, Ng CC, Too CL, Choon SE, Lee CK, Chung WH, et al. Association of HLA-B*15:13 and HLA-B*15:02 with phenytoin-induced severe cutaneous adverse reactions in a Malay population. Pharmacogenomics J. 2017; 17:170–173. DOI: 10.1038/tpj.2016.10. PMID: 26927288.
Article8. Mansur AT, Pekcan Yaşar S, Göktay F. Anticonvulsant hypersensitivity syndrome: Clinical and laboratory features. Int J Dermatol. 2008; 47:1184–1189. DOI: 10.1111/j.1365-4632.2008.03827.x. PMID: 18986457.
Article9. Romano A, Torres MJ, Castells M, Sanz ML, Blanca M. Diagnosis and management of drug hypersensitivity reactions. J Allergy Clin Immunol. 2011; 127:S67–S73. DOI: 10.1016/j.jaci.2010.11.047. PMID: 21354502.
Article10. Kim EY, Seol JE, Choi JH, Kim NY, Shin JK. Allopurinol-induced severe cutaneous adverse reactions: A report of three cases with the HLA-B* 58:01 allele who underwent lymphocyte activation test. Transl Clin Pharmacol. 2017; 25:63–66.11. World Health Organization (WHO), Uppsala Monitoring Centre. The use of the WHO-UMC system for standardised case causality assessment. Accessed 30 May 2019. http://www.who.int/medicines/areas/quality_safety/safety_efficacy/WHOcausality_assessment.pdf.12. In JW, Roh EY, Oh S, Shin S, Park KU, Song EY. Allele and haplotype frequencies of human leukocyte antigen-A, -B, -C, -DRB1, and -DQB1 from sequence-based DNA typing data in Koreans. Ann Lab Med. 2015; 35:429–435. DOI: 10.3343/alm.2015.35.4.429. PMID: 26131415.
Article13. Kim EY, Ji KH, Kim HJ, Jung HE, Cha EY, Shin J. HLA-A*24:02/B*51:01 haplotype and lamotrigine-induced cutaneous adverse drug reactions in Koreans. Transl Clin Pharmacol. 2016; 24:143–146.14. Ghosh K, Banerjee G, Ghosal AK, Nandi J. Cutaneous drug hypersensitivity: Immunological and genetic perspective. Indian J Dermatol. 2011; 56:137–144. DOI: 10.4103/0019-5154.80402. PMID: 21716938.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Allopurinol-induced severe cutaneous adverse reactions: A report of three cases with the HLA-B*58:01 allele who underwent lymphocyte activation test
- Hypersensitivity to Anticonvulsants
- Absence of HLA-B*1502 and HLA-A*3101 Alleles in 9 Korean Patients With Antiepileptic Drug-Induced Skin Rash: A Preliminary Study
- HLA-A*24:02/B*51:01 haplotype and lamotrigine-induced cutaneous adverse drug reactions in Koreans
- Cutaneous adverse drug reactions