Int J Stem Cells.
2018 Jun;11(1):39-47. 10.15283/ijsc17074.
Local Mesenchymal Stem Cell Therapy in Experimentally Induced Colitis in the Rat
- Affiliations
-
- 1Department of General and Digestive Surgery, Unit Colorectal Surgery, “Virgen del RocÃo†University Hospital/IBiS/CSIC/University of Seville, Seville, Spain. fportilla@us.es
- 2Institute of Biomedicine of Seville (IBiS), “Virgen del RocÃo†University Hospital/IBiS/CSIC/University of Seville, Seville, Spain.
- 3National Institute of Toxicology and Forensic Sciences, Seville, Spain.
- KMID: 2413499
- DOI: http://doi.org/10.15283/ijsc17074
Abstract
- BACKGROUND
Multipotent mesenchymal stem cells (MSCs) have been used in inflammatory bowel diseases because of their immunomodulatory and regenerative properties. We investigated their local use in an experimental model of colitis in the rat.
MATERIALS AND METHODS
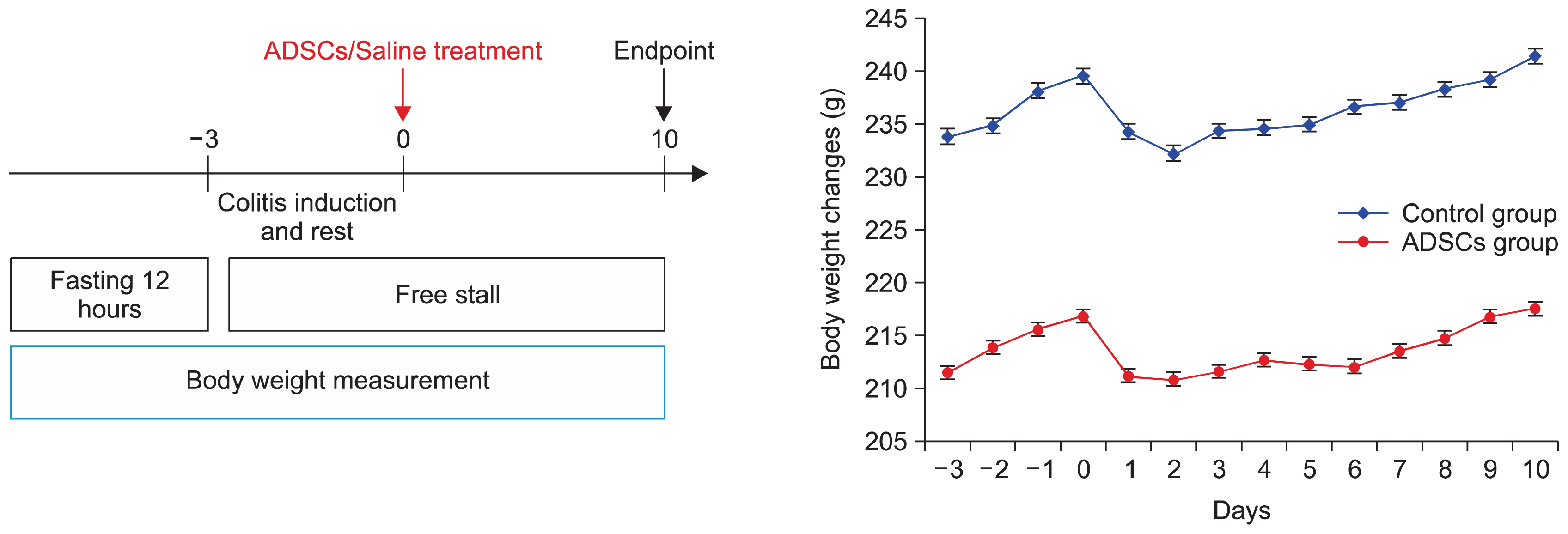
Colitis was induced into 20 Wistar rats with local TNBS instillation. Allogeneic stem cells were derived from rat adipose tissue and labeled with PKH2 linker dye with creation of a control and a second group treated by a local injection into the rectal wall of 2×106 allogeneic adipose tissue-derived stem cells (ADSCs). The thicknesses of different components of the rectum were measured with comparisons made in different parts of the colon of the Hunter inflammatory score. PKH2-dyed ADSCs were detected by fluorescence microscopy.
RESULTS
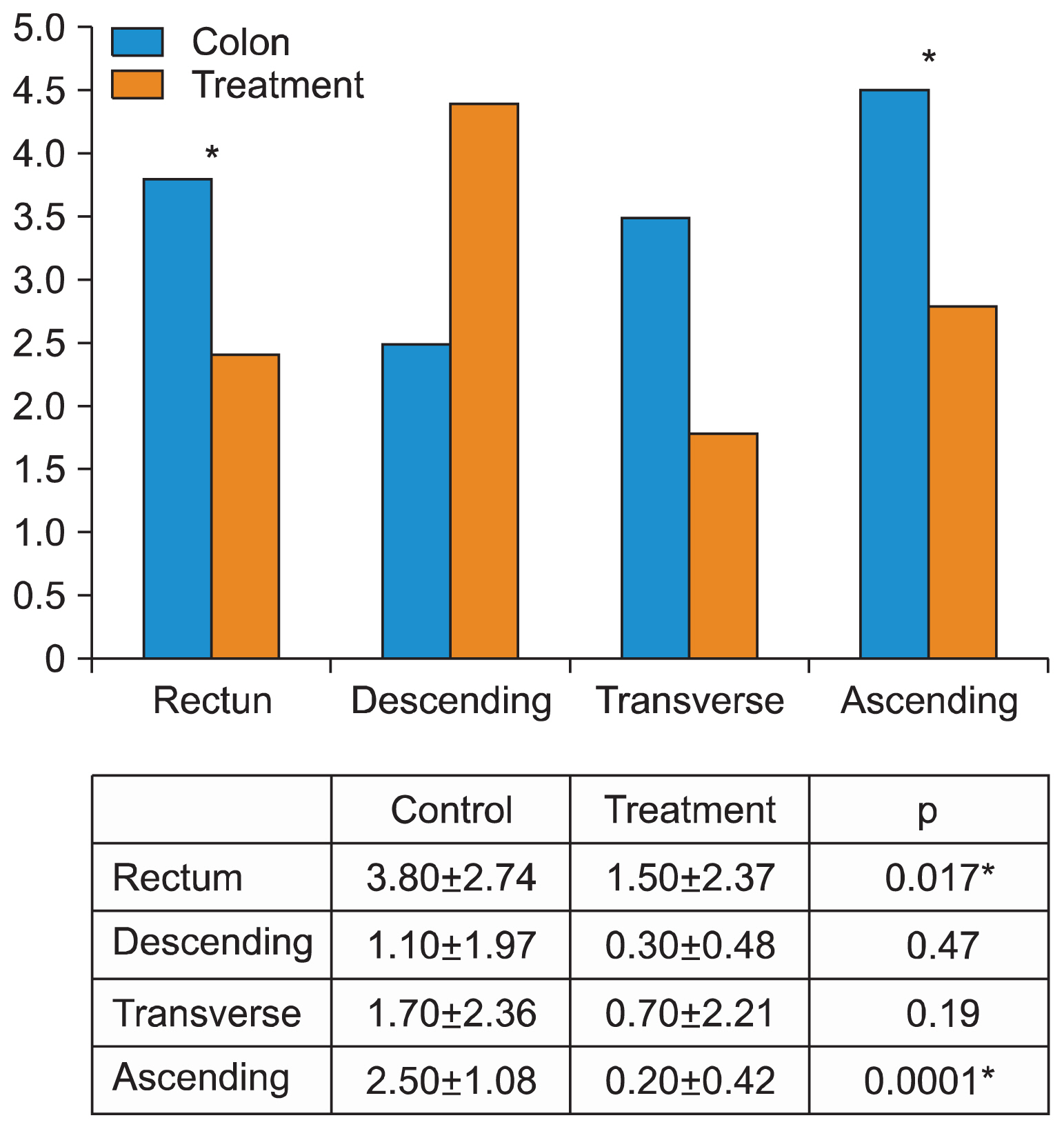
AND CONCLUSIONS: Total colitis was induced in 19/20 rats with homing of fluorescent ADSCs. to the crypt base and perivascular space of the submucosa. There were no differences in component rectal wall thicknesses with a higher Hunter score in the treated group compared with the controls, in the rectum (3.8±2.74 vs. 1.5±2.37, respectively; p=0.017) and in right colon (2.5±1.08 vs. 0.20±0.42, respectively; p=0.0001). Local colonic injection of allogeneic adipose stem cells. in experimental colitis is feasible and safe. There is demonstrable homing of cells in chemically-induced colitis both to the treated region and parts of the colon distant to the MSC treatment site. Such cells readily proliferate in vitro and could potentially be a source for future treatment of resistant disease.
MeSH Terms
Figure
Reference
-
References
1. Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015; 12:720–727. DOI: 10.1038/nrgastro.2015.150. PMID: 26323879.
Article2. Kellermayer R. Challenges for epigenetic research in inflammatory bowel diseases. Epigenomics. 2017; 9:527–538. DOI: 10.2217/epi-2016-0155. PMID: 28343422.
Article3. Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nuñez G, Cho JH. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001; 411:603–606. DOI: 10.1038/35079114. PMID: 11385577.
Article4. Valatas V, Bamias G, Kolios G. Experimental colitis models: Insights into the pathogenesis of inflammatory bowel disease and translational issues. Eur J Pharmacol. 2015; 759:253–264. DOI: 10.1016/j.ejphar.2015.03.017. PMID: 25814256.
Article5. Cominelli F, Arseneau KO, Rodriguez-Palacios A, Pizarro TT. Uncovering pathogenic mechanisms of inflammatory bowel disease using mouse models of Crohn’s disease-like ileitis: what is the right model? Cell Mol Gastroenterol Hepatol. 2017; 4:19–32. DOI: 10.1016/j.jcmgh.2017.02.010. PMID: 28560286. PMCID: 5439236.
Article6. Stagg J, Galipeau J. Mechanisms of immune modulation by mesenchymal stromal cells and clinical translation. Curr Mol Med. 2013; 13:856–867. DOI: 10.2174/1566524011313050016. PMID: 23642066.
Article7. Hoogduijn MJ, Betjes MG, Baan CC. Mesenchymal stromal cells for organ transplantation: different sources and unique characteristics? Curr Opin Organ Transplant. 2014; 19:41–46. DOI: 10.1097/MOT.0000000000000036.8. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8:315–317. DOI: 10.1080/14653240600855905. PMID: 16923606.
Article9. Krampera M, Galipeau J, Shi Y, Tarte K, Sensebe L. MSC Committee of the International Society for Cellular Therapy (ISCT). Immunological characterization of multipotent mesenchymal stromal cells--The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy. 2013; 15:1054–1061. DOI: 10.1016/j.jcyt.2013.02.010. PMID: 23602578.
Article10. Kashyap A, Forman SJ. Autologous bone marrow transplantation for non-Hodgkin’s lymphoma resulting in longterm remission of coincidental Crohn’s disease. Br J Haematol. 1998; 103:651–652. DOI: 10.1046/j.1365-2141.1998.01059.x. PMID: 9858212.
Article11. Onken J, Gallup D, Hanson J, Pandak M, Custer L. Successful outpatient treatment of refractory Crohn’s disease using adult mesenchymal stem cells (Abstract). In : American College of Gastroenterology Conference Annual Meeting; Las Vegas. October 2006; Available from: http://universe.gi.org/contentitem.asp?c=1047.12. Hawkey CJ, Hommes DW. Is stem cell therapy ready for prime time in treatment of inflammatory bowel diseases? Gastroenterology. 2017; 152:389–397.e2. DOI: 10.1053/j.gastro.2016.11.003.
Article13. de la Portilla F, Alba F, García-Olmo D, Herrerías JM, González FX, Galindo A. Expanded allogeneic adipose- derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: results from a multicenter phase I/IIa clinical trial. Int J Colorectal Dis. 2013; 28:313–323. DOI: 10.1007/s00384-012-1581-9.
Article14. Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989; 96:795–803. DOI: 10.1016/S0016-5085(89)80079-4.
Article15. Pfeiffer CJ, Sato S, Qiu BS, Keith JC, Evangelista S. Cellular pathology of experimental colitis induced by trinitrobenzenesulphonic acid (TNBS): protective effects of recombinant human interleukin-11. Inflammopharmacology. 1997; 5:363–381. DOI: 10.1007/s10787-997-0033-6. PMID: 17657615.
Article16. Millar AD, Rampton DS, Chander CL, Claxson AW, Blades S, Coumbe A, Panetta J, Morris CJ, Blake DR. Evaluating the antioxidant potential of new treatments for inflammatory bowel disease using a rat model of colitis. Gut. 1996; 39:407–415. DOI: 10.1136/gut.39.3.407. PMID: 8949646. PMCID: 1383348.
Article17. Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013; 15:641–648. DOI: 10.1016/j.jcyt.2013.02.006. PMID: 23570660. PMCID: 3979435.
Article18. Boquest AC, Shahdadfar A, Brinchmann JE, Collas P. Isolation of stromal stem cells from human adipose tissue. Methods Mol Biol. 2006; 325:35–46. PMID: 16761717.
Article19. Yoshimura K, Shigeura T, Matsumoto D, Sato T, Takaki Y, Aiba-Kojima E, Sato K, Inoue K, Nagase T, Koshima I, Gonda K. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006; 208:64–76. DOI: 10.1002/jcp.20636. PMID: 16557516.
Article20. Zhu Y, Liu T, Song K, Fan X, Ma X, Cui Z. Adipose-derived stem cell: a better stem cell than BMSC. Cell Biochem Funct. 2008; 26:664–675. DOI: 10.1002/cbf.1488. PMID: 18636461.
Article21. Poon RY, Ohlsson-Wilhelm BM, Bagwell CB, Muirhead KA. Use of PKH membrane intercalating dyes to monitor cell trafficking and function. Diamond RA, DeMaggio S, editors. Living Color: Protocols in Flow Cytometry and Cell Sorting. New York, NY: Springer-Verlag;2000. p. 302–352. DOI: 10.1007/978-3-642-57049-0_26.
Article22. Hunter MM, Wang A, Hirota CL, McKay DM. Neutralizing anti-IL-10 antibody blocks the protective effect of tapeworm infection in a murine model of chemically induced colitis. J Immunol. 2005; 174:7368–7375. DOI: 10.4049/jimmunol.174.11.7368. PMID: 15905584.
Article23. Chassaing B, Darfeuille-Michaud A. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011; 140:1720–1728. DOI: 10.1053/j.gastro.2011.01.054. PMID: 21530738.
Article24. Grégoire C, Lechanteur C, Briquet A, Baudoux É, Baron F, Louis E, Beguin Y. Review article: mesenchymal stromal cell therapy for inflammatory bowel diseases. Aliment Pharmacol Ther. 2017; 45:205–221. DOI: 10.1111/apt.13864.
Article25. Randhawa PK, Singh K, Singh N, Jaggi AS. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J Physiol Pharmacol. 2014; 18:279–288. DOI: 10.4196/kjpp.2014.18.4.279. PMID: 25177159. PMCID: 4146629.
Article26. Motavallian-Naeini A, Andalib S, Rabbani M, Mahzouni P, Afsharipour M, Minaiyan M. Validation and optimization of experimental colitis induction in rats using 2, 4, 6-trinitrobenzene sulfonic acid. Res Pharm Sci. 2012; 7:159–169. PMID: 23181094. PMCID: 3501925.27. Fawzy SA, El-Din Abo-Elnou RK, Abd-El-Maksoud El-Deeb DF, Yousry Abd-Elkader MM. The possible role of mesenchymal stem cells therapy in the repair of experimentally induced colitis in male albino rats. Int J Stem Cells. 2013; 6:92–103. DOI: 10.15283/ijsc.2013.6.2.92.
Article28. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001; 7:211–228. DOI: 10.1089/107632701300062859. PMID: 11304456.
Article29. García-Gómez I, Elvira G, Zapata AG, Lamana ML, Ramírez M, Castro JG, Arranz MG, Vicente A, Bueren J, García-Olmo D. Mesenchymal stem cells: biological properties and clinical applications. Expert Opin Biol Ther. 2010; 10:1453–1468. DOI: 10.1517/14712598.2010.519333. PMID: 20831449.
Article30. Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003; 101:3722–3729. DOI: 10.1182/blood-2002-07-2104.
Article31. Hawkey CJ, Allez M, Clark MM, Labopin M, Lindsay JO, Ricart E, Rogler G, Rovira M, Satsangi J, Danese S, Russell N, Gribben J, Johnson P, Larghero J, Thieblemont C, Ardizzone S, Dierickx D, Ibatici A, Littlewood T, Onida F, Schanz U, Vermeire S, Colombel JF, Jouet JP, Clark E, Saccardi R, Tyndall A, Travis S, Farge D. Autologous hematopoetic stem cell transplantation for refractory crohn disease: a randomized clinical trial. JAMA. 2015; 314:2524–2534. DOI: 10.1001/jama.2015.16700. PMID: 26670970.
Article32. Yanai H, Hanauer SB. Assessing response and loss of response to biological therapies in IBD. Am J Gastroenterol. 2011; 106:685–698. DOI: 10.1038/ajg.2011.103. PMID: 21427713.
Article33. Panés J, García-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, Dignass A, Nachury M, Ferrante M, Kazemi-Shirazi L, Grimaud JC, de la Portilla F, Goldin E, Richard MP, Leselbaum A, Danese S. ADMIRE CD Study Group Collaborators. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016; 388:1281–1290. DOI: 10.1016/S0140-6736(16)31203-X.
Article34. Mao F, Tu Q, Wang L, Chu F, Li X, Li HS, Xu W. Mesenchymal stem cells and their therapeutic applications in inflammatory bowel disease. Oncotarget. 2017; 8:38008–38021. DOI: 10.18632/oncotarget.16682. PMID: 28402942. PMCID: 5514968.
Article35. Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009; 4:206–216. DOI: 10.1016/j.stem.2009.02.001. PMID: 19265660.
Article36. Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007; 25:2648–2659. DOI: 10.1634/stemcells.2007-0226. PMID: 17615264.
Article37. Wang M, Liang C, Hu H, Zhou L, Xu B, Wang X, Han Y, Nie Y, Jia S, Liang J, Wu K. Intraperitoneal injection (IP), Intravenous injection (IV) or anal injection (AI)? Best way for mesenchymal stem cells transplantation for colitis. Sci Rep. 2016; 6:30696. DOI: 10.1038/srep30696. PMID: 27488951. PMCID: 4973258.
Article38. Schoeb TR, Bullard DC. Microbial and histopathologic considerations in the use of mouse models of inflammatory bowel diseases. Inflamm Bowel Dis. 2012; 18:1558–1565. DOI: 10.1002/ibd.22892. PMID: 22294506. PMCID: 3733552.
Article39. Kim N, Cho SG. New strategies for overcoming limitations of mesenchymal stem cell-based immune modulation. Int J Stem Cells. 2015; 8:54–68. DOI: 10.15283/ijsc.2015.8.1.54. PMID: 26019755. PMCID: 4445710.
Article40. Moll G, Alm JJ, Davies LC, von Bahr L, Heldring N, Stenbeck-Funke L, Hamad OA, Hinsch R, Ignatowicz L, Locke M, Lönnies H, Lambris JD, Teramura Y, Nilsson-Ekdahl K, Nilsson B, Le Blanc K. Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells. 2014; 32:2430–2442. DOI: 10.1002/stem.1729. PMID: 24805247. PMCID: 4381870.
Article41. Romieu-Mourez R, Coutu DL, Galipeau J. The immune plasticity of mesenchymal stromal cells from mice and men: concordances and discrepancies. Front Biosci (Elite Ed). 2012; 4:824–837.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Possible Role of Mesenchymal Stem Cells Therapy in the Repair of Experimentally Induced Colitis in Male Albino Rats
- Combination Cell Therapy with Mesenchymal Stem Cells and Neural Stem Cells for Brain Stroke in Rats
- Clinical utilization of cord blood over human health: experience of stem cell transplantation and cell therapy using cord blood in Korea
- Current Trends and Prospect of Cell Therapy using Hematopoietic Stem Cells
- The hope and hype of stem cell therapy