J Gynecol Oncol.
2011 Dec;22(4):225-232. 10.3802/jgo.2011.22.4.225.
Combination of imatinib mesylate with lithium chloride and medroxyprogesterone acetate is highly active in Ishikawa endometrial carcinoma in vitro
- Affiliations
-
- 1Department of Histology and Embryology, Istanbul University Faculty of Medicine, Istanbul, Turkey. bilira@istanbul.edu.tr
- 2Department of Biochemistry, Yeni Yuzyil University Faculty of Medicine, Istanbul, Turkey.
- 3Department of Molecular Biology and Genetics, Ankara University Faculty of Medicine, Ankara, Turkey.
- 4Department of Pathophysiology, Ankara University Faculty of Medicine, Ankara, Turkey.
- KMID: 2173623
- DOI: http://doi.org/10.3802/jgo.2011.22.4.225
Abstract
OBJECTIVE
The aim of the study was to investigate whether lithium chloride and medroxyprogesterone acetate can potentiate the cytotoxicity of imatinib mesylate in human endometrial cancer in vitro and the effect of midkine in these therapies.
METHODS
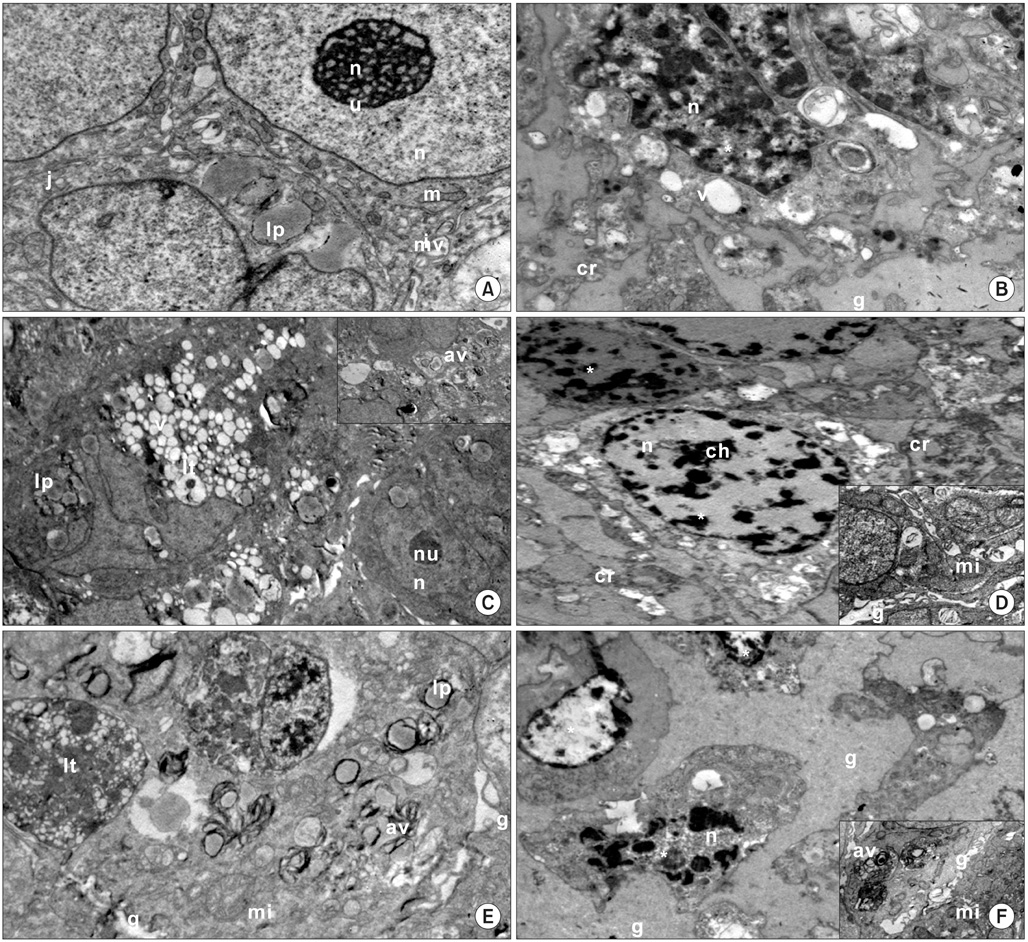
Imatinib mesylate (50 microM), lithium chloride (100 microM), medroxyprogesterone acetate (200 microM) and their combination were applied to monolayer and three dimensional cultures of human Ishikawa endometrial cancer for 72 hours. The cell proliferation index, apoptotic index, caspase-3 and midkine levels, cell cycle distributions in monolayer cultures and cell ultrastructure in spheroid cultures were evaluated. Results were statistically analyzed using the Student's t-test.
RESULTS
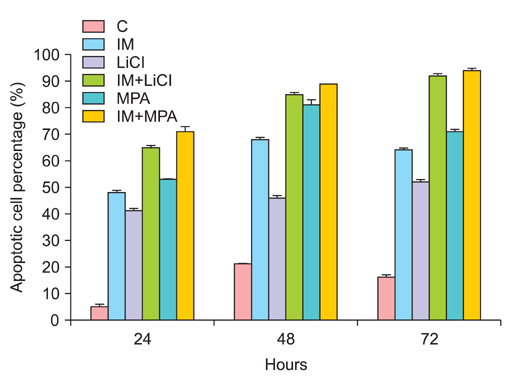
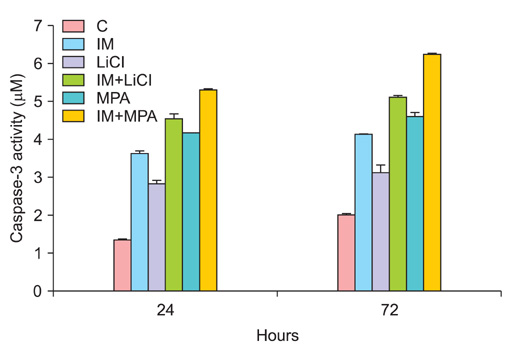
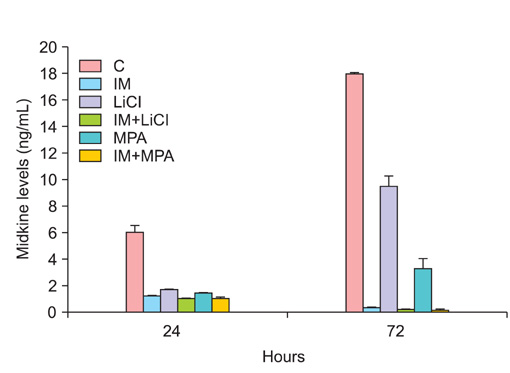
All drug applications inhibited cell proliferation (p<0.05), however the combination were the effective groups for 72 hours (p<0.05). Interestingly, although the loss of efficiency was seen higly seen every 24 hours at single applications, the inhibition rates of the combination groups were almost same for 72 hours. In concordance with these results, the apoptotic index, caspase-3 levels (p<0.05), cell morphology and ultrastructure damages were much higher in the combination groups. Imatinib mesylate induced S-phase arrest, however other groups induced G0+G1-phase arrest at 24 hours and all groups induced G0+G1 arrest at 72 hours (p<0.05). Imatinib mesylate and imatinib mesylate with medroxyprogesterone acetate induced highest decrease in midkine levels, respectively (p<0.05).
CONCLUSION
The present study showed that the combination of imatinib mesylate with lithium chloride and medroxyprogesterone acetate is highly active in Ishikawa endometrial carcinoma in vitro and the inhibition of midkine involved in their mechanism of action against endometrium defense.
Keyword
MeSH Terms
-
Benzamides
Caspase 3
Cell Cycle
Cell Proliferation
Cytokines
Endometrial Neoplasms
Endometrium
Female
Humans
Imatinib Mesylate
Lithium
Lithium Chloride
Medroxyprogesterone
Medroxyprogesterone Acetate
Mesylates
Piperazines
Pyrimidines
Benzamides
Caspase 3
Cytokines
Lithium
Lithium Chloride
Medroxyprogesterone
Medroxyprogesterone Acetate
Mesylates
Piperazines
Pyrimidines
Figure
Reference
-
1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009. 59:225–249.2. Di Cristofano A, Ellenson LH. Endometrial carcinoma. Annu Rev Pathol. 2007. 2:57–85.3. Abal M, Planaguma J, Gil-Moreno A, Monge M, Gonzalez M, Baro T, et al. Molecular pathology of endometrial carcinoma: transcriptional signature in endometrioid tumors. Histol Histopathol. 2006. 21:197–204.4. Lax SF. Molecular genetic pathways in various types of endometrial carcinoma: from a phenotypical to a molecular-based classification. Virchows Arch. 2004. 444:213–223.5. Waller CF. Imatinib mesylate. Recent Results Cancer Res. 2010. 184:3–20.6. Salvatierra A, Tarrats A, Gomez C, Sastre JM, Balana C. A case of c-kit positive high-grade stromal endometrial sarcoma responding to Imatinib Mesylate. Gynecol Oncol. 2006. 101:545–547.7. Mitsuhashi T, Nakayama M, Sakurai S, Fujimura M, Shimizu Y, Ban S, et al. KIT-negative undifferentiated endometrial sarcoma with the amplified epidermal growth factor receptor gene showing a temporary response to imatinib mesylate. Ann Diagn Pathol. 2007. 11:49–54.8. Ha HT, Lee JS, Urba S, Koenig RJ, Sisson J, Giordano T, et al. A phase II study of imatinib in patients with advanced anaplastic thyroid cancer. Thyroid. 2010. 20:975–980.9. Ustach CV, Huang W, Conley-LaComb MK, Lin CY, Che M, Abrams J, et al. A novel signaling axis of matriptase/PDGF-D/β-PDGFR in human prostate cancer. Cancer Res. 2010. 70:9631–9640.10. Vidal F, de Araujo WM, Cruz AL, Tanaka MN, Viola JP, Morgado-Diaz JA. Lithium reduces tumorigenic potential in response to EGF signaling in human colorectal cancer cells. Int J Oncol. 2011. 38:1365–1373.11. Cho YJ, Kim JH, Yoon J, Cho SJ, Ko YS, Park JW, et al. Constitutive activation of glycogen synthase kinase-3beta correlates with better prognosis and cyclin-dependent kinase inhibitors in human gastric cancer. BMC Gastroenterol. 2010. 10:91.12. Bilir A, Erguven M, Yazihan N, Aktas E, Oktem G, Sabanci A. Enhancement of vinorelbine-induced cytotoxicity and apoptosis by clomipramine and lithium chloride in human neuroblastoma cancer cell line SH-SY5Y. J Neurooncol. 2010. 100:385–395.13. Zhu H, Han B, Pan X, Qi H, Xu L. Thiazolidenediones induce tumour-cell apoptosis through the Akt-GSK3β pathway. J Clin Pharm Ther. 2011. 03. 16. [Epub]. http://dx.doi.org/10.1111/j.1365-2710.2011.01251.x.14. Nishio S, Koyanagi T, Miyabe K, Kuromatsu H. Two cases of multidrug-resistant recurrent endometrial cancer successfully treated with medroxyprogesterone acetate (MPA). Gan To Kagaku Ryoho. 2010. 37:735–738.15. Cade TJ, Quinn MA, Rome RM, Neesham D. Progestogen treatment options for early endometrial cancer. BJOG. 2010. 117:879–884.16. Madeddu C, Maccio A, Panzone F, Tanca FM, Mantovani G. Medroxyprogesterone acetate in the management of cancer cachexia. Expert Opin Pharmacother. 2009. 10:1359–1366.17. Yang S, Thiel KW, Leslie KK. Progesterone: the ultimate endometrial tumor suppressor. Trends Endocrinol Metab. 2011. 22:145–152.18. Wang Y, van der Zee M, Fodde R, Blok LJ. Wnt/B-catenin and sex hormone signaling in endometrial homeostasis and cancer. Oncotarget. 2010. 1:674–684.19. Muramatsu T. Midkine, a heparin-binding cytokine with multiple roles in development, repair and diseases. Proc Jpn Acad Ser B Phys Biol Sci. 2010. 86:410–425.20. Zhang L, Rees MC, Bicknell R. The isolation and long-term culture of normal human endometrial epithelium and stroma: expression of mRNAs for angiogenic polypeptides basally and on oestrogen and progesterone challenges. J Cell Sci. 1995. 108:323–331.21. Miller MC 3rd, Johnson KR, Willingham MC, Fan W. Apoptotic cell death induced by baccatin III, a precursor of paclitaxel, may occur without G(2)/M arrest. Cancer Chemother Pharmacol. 1999. 44:444–452.22. Bilir A, Altinoz MA, Erkan M, Ozmen V, Aydiner A. Autophagy and nuclear changes in FM3A breast tumor cells after epirubicin, medroxyprogesterone and tamoxifen treatment in vitro. Pathobiology. 2001. 69:120–126.23. Erguven M, Yazihan N, Aktas E, Sabanci A, Li CJ, Oktem G, et al. Carvedilol in glioma treatment alone and with imatinib in vitro. Int J Oncol. 2010. 36:857–866.24. Orrenius S, Nicotera P, Zhivotovsky B. Cell death mechanisms and their implications in toxicology. Toxicol Sci. 2011. 119:3–19.25. Rawnaq T, Kunkel M, Bachmann K, Simon R, Zander H, Brandl S, et al. Serum midkine correlates with tumor progression and imatinib response in gastrointestinal stromal tumors. Ann Surg Oncol. 2011. 18:559–565.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In vitro respones of gynecological cancer cell lines to the GnRH agonist, medroxyprogesterone acetate and tamoxifen
- A Case of Lichenoid Drug Eruption Associated with Imatinib Mesylate
- Marked Regression of Bilateral Pulmonary Metastases: Report of A Case Renal Cell Carcinoma after Nephrectomy and Medroxyprogesterone Acetate Therapy
- Renal and hormonal effects of lithium chloride
- Treatment efficacy of high dose progestin in young women with early stage of endometrial carcinoma