Blood Res.
2015 Jun;50(2):80-86. 10.5045/br.2015.50.2.80.
Down-regulation of the autophagy gene, ATG7, protects bone marrow-derived mesenchymal stem cells from stressful conditions
- Affiliations
-
- 1Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran. roudkenar@ibto.ir
- 2Department of Anatomical Sciences, School of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran.
- KMID: 2172771
- DOI: http://doi.org/10.5045/br.2015.50.2.80
Abstract
- BACKGROUND
Mesenchymal stem cells (MSCs) are valuable for cell-based therapy. However, their application is limited owing to their low survival rate when exposed to stressful conditions. Autophagy, the process by which cells recycle the cytoplasm and dispose of defective organelles, is activated by stress stimuli to adapt, tolerate adverse conditions, or trigger the apoptotic machinery. This study aimed to determine whether regulation of autophagy would affect the survival of MSCs under stress conditions.
METHODS
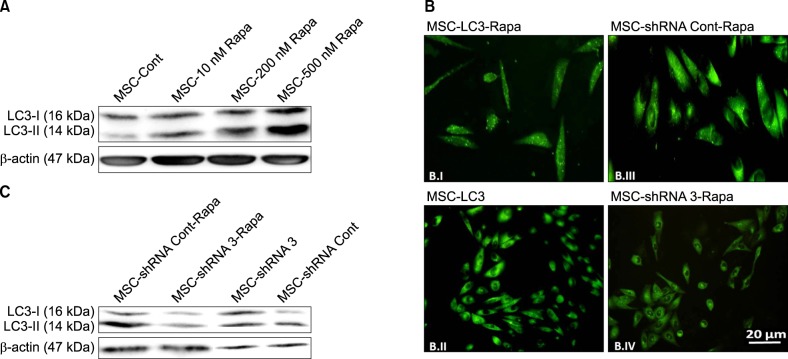
Autophagy was induced in bone marrow-derived MSCs (BM-MSCs) by rapamycin, and was inhibited via shRNA-mediated knockdown of the autophagy specific gene, ATG7. ATG7 expression in BM-MSCs was evaluated by reverse transcription polymerase chain reaction (RT-PCR), western blot, and quantitative PCR (qPCR). Cells were then exposed to harsh microenvironments, and a water-soluble tetrazolium salt (WST)-1 assay was performed to determine the cytotoxic effects of the stressful conditions on cells.
RESULTS
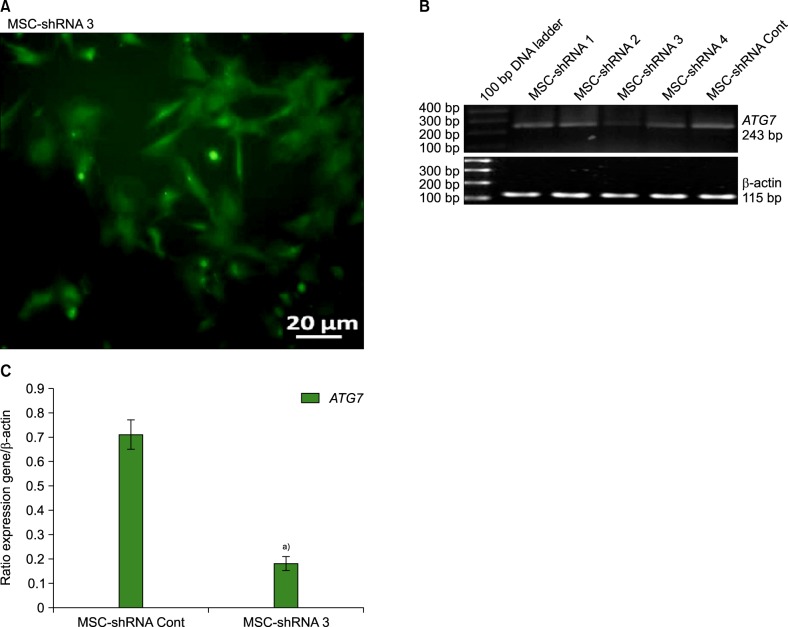
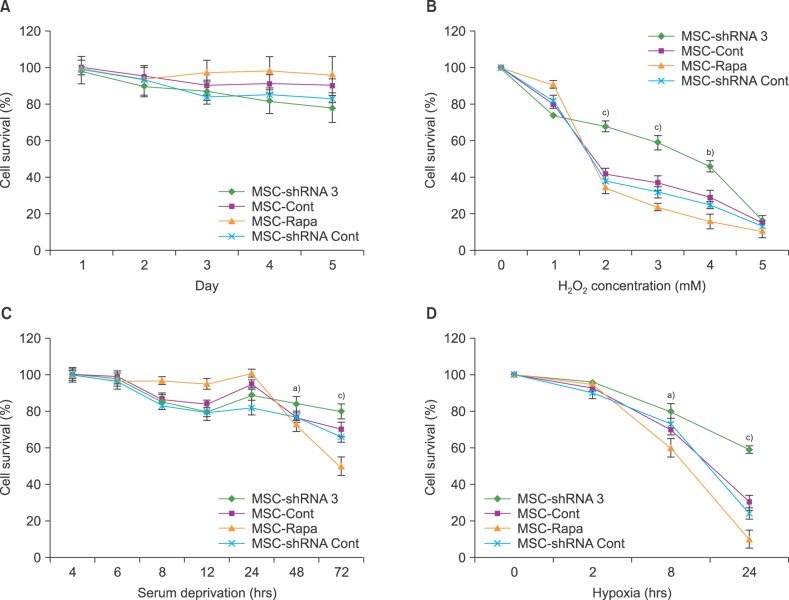
Of 4 specific ATG7-inhibitor clones analyzed, only shRNA clone 3 decreased ATG7 expression. Under normal conditions, the induction of autophagy slightly increased the viability of MSCs while autophagy inhibition decreased their viability. However, under stressful conditions such as hypoxia, serum deprivation, and oxidative stress, the induction of autophagy resulted in cell death, while its inhibition potentiated MSCs to withstand the stress conditions. The viability of autophagy-suppressed MSCs was significantly higher than that of relevant controls (P<0.05, P<0.01 and P<0.001).
CONCLUSION
Autophagy modulation in MSCs can be proposed as a new strategy to improve their survival rate in stressful microenvironments.
MeSH Terms
Figure
Reference
-
1. Wang S, Qu X, Zhao RC. Clinical applications of mesenchymal stem cells. J Hematol Oncol. 2012; 5:19. PMID: 22546280.
Article2. Al-Nbaheen M, Vishnubalaji R, Ali D, et al. Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential. Stem Cell Rev. 2013; 9:32–43. PMID: 22529014.
Article3. Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013; 45:e54. PMID: 24232253.
Article4. Yoon DS, Kim YH, Jung HS, Paik S, Lee JW. Importance of Sox2 in maintenance of cell proliferation and multipotency of mesenchymal stem cells in low-density culture. Cell Prolif. 2011; 44:428–440. PMID: 21951286.
Article5. Haider HKh, Ashraf M. Strategies to promote donor cell survival: combining preconditioning approach with stem cell transplantation. J Mol Cell Cardiol. 2008; 45:554–566. PMID: 18561945.
Article6. Wei H, Li Z, Hu S, Chen X, Cong X. Apoptosis of mesenchymal stem cells induced by hydrogen peroxide concerns both endoplasmic reticulum stress and mitochondrial death pathway through regulation of caspases, p38 and JNK. J Cell Biochem. 2010; 111:967–978. PMID: 20665666.
Article7. Amiri F, Jahanian-Najafabadi A, Roudkenar MH. In vitro augmentation of mesenchymal stem cells viability in stressful microenvironments : In vitro augmentation of mesenchymal stem cells viability. Cell Stress Chaperones. 2015; 20:237–251. PMID: 25527070.8. Mizushima N. Autophagy: process and function. Genes Dev. 2007; 21:2861–2873. PMID: 18006683.
Article9. Shen HM, Mizushima N. At the end of the autophagic road: an emerging understanding of lysosomal functions in autophagy. Trends Biochem Sci. 2014; 39:61–71. PMID: 24369758.
Article10. Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010; 221:3–12. PMID: 20225336.
Article11. Yu L, Lenardo MJ, Baehrecke EH. Autophagy and caspases: a new cell death program. Cell Cycle. 2004; 3:1124–1126. PMID: 15326383.
Article12. Boya P, González-Polo RA, Casares N, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005; 25:1025–1040. PMID: 15657430.
Article13. Lee IH, Cao L, Mostoslavsky R, et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A. 2008; 105:3374–3379. PMID: 18296641.
Article14. Thorburn J, Andrysik Z, Staskiewicz L, et al. Autophagy controls the kinetics and extent of mitochondrial apoptosis by regulating PUMA levels. Cell Rep. 2014; 7:45–52. PMID: 24685133.
Article15. Guan JL, Simon AK, Prescott M, et al. Autophagy in stem cells. Autophagy. 2013; 9:830–849. PMID: 23486312.
Article16. Hamedi-Asl P, Halabian R, Bahmani P, et al. Adenovirus-mediated expression of the HO-1 protein within MSCs decreased cytotoxicity and inhibited apoptosis induced by oxidative stresses. Cell Stress Chaperones. 2012; 17:181–190. PMID: 21993906.
Article17. Tanida I, Ueno T, Kominami E. LC3 and autophagy. Methods Mol Biol. 2008; 445:77–88. PMID: 18425443.
Article18. Zhu W, Chen J, Cong X, Hu S, Chen X. Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Stem Cells. 2006; 24:416–425. PMID: 16253984.
Article19. Park HR, Tomida A, Sato S, et al. Effect on tumor cells of blocking survival response to glucose deprivation. J Natl Cancer Inst. 2004; 96:1300–1310. PMID: 15339968.
Article20. Baehrecke EH. Autophagy: dual roles in life and death? Nat Rev Mol Cell Biol. 2005; 6:505–510. PMID: 15928714.
Article21. Kuwahara Y, Oikawa T, Ochiai Y, et al. Enhancement of autophagy is a potential modality for tumors refractory to radiotherapy. Cell Death Dis. 2011; 2:e177. PMID: 21716292.
Article22. Han X, Liu JX, Li XZ. Salvianolic acid B inhibits autophagy and protects starving cardiac myocytes. Acta Pharmacol Sin. 2011; 32:38–44. PMID: 21113177.
Article23. Yu SW, Baek SH, Brennan RT, et al. Autophagic death of adult hippocampal neural stem cells following insulin withdrawal. Stem Cells. 2008; 26:2602–2610. PMID: 18653772.
Article24. Hou J, Han ZP, Jing YY, et al. Autophagy prevents irradiation injury and maintains stemness through decreasing ROS generation in mesenchymal stem cells. Cell Death Dis. 2013; 4:e844. PMID: 24113178.
Article25. Mortensen M, Soilleux EJ, Djordjevic G, et al. The autophagy protein Atg7 is essential for hematopoietic stem cell maintenance. J Exp Med. 2011; 208:455–467. PMID: 21339326.
Article26. Song C, Song C, Tong F. Autophagy induction is a survival response against oxidative stress in bone marrow-derived mesenchymal stromal cells. Cytotherapy. 2014; 16:1361–1370.
Article27. Codogno P, Meijer AJ. Atg5: more than an autophagy factor. Nat Cell Biol. 2006; 8:1045–1047. PMID: 17013414.
Article28. Hou H, Zhang Y, Huang Y, et al. Inhibitors of phosphatidylinositol 3'-kinases promote mitotic cell death in HeLa cells. PLoS One. 2012; 7:e35665. PMID: 22545128.
Article29. Kanematsu S, Uehara N, Miki H, et al. Autophagy inhibition enhances sulforaphane-induced apoptosis in human breast cancer cells. Anticancer Res. 2010; 30:3381–3390. PMID: 20944112.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bone marrow-derived stem cells contribute to regeneration of the endometrium
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Clinical Safety and Efficacy of Autologous Bone Marrow-Derived Mesenchymal Stem Cell Transplantation in Sensorineural Hearing Loss Patients
- Differential Potential of Stem Cells Following Their Origin: Subacromial Bursa, Bone Marrow, Umbilical Cord Blood
- Repopulation of autophagy-deficient stromal cells with autophagy-intact cells after repeated breeding in uterine mesenchyme-specific Atg7 knockout mice