Nutr Res Pract.
2008 Dec;2(4):234-239. 10.4162/nrp.2008.2.4.234.
Pre- and post-initiation modulating effects of green tea ingestion on rat hepatocarcinogenesis
- Affiliations
-
- 1Pulmuone Holdings Co. Ltd., Seoul 120-600, Korea.
- 2Department of Food Science and Nutrition, Soonchunhyang University, Asan, Chungnam 336-745, Korea. hskim1@sch.ac.kr
- 3Department of Food Science and Nutrition, Seoul National University, Gwanak 599 Gwanak-ro, Gwanak-gu, Seoul 151-742, Korea.
- KMID: 2139197
- DOI: http://doi.org/10.4162/nrp.2008.2.4.234
Abstract
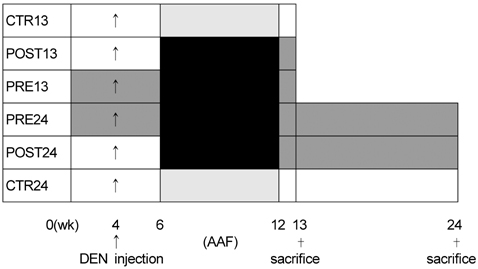
- The purpose of this study was to investigate the effects of green tea ingestion on hepatocarcinogenesis before and after its initiation. Male Sprague-Dawley rats were fed an AIN76A diet with or without green tea. Initiation was induced by a single dose (200 mg/kg) of diethylnitrosamine at week 4 and 0.02% (w/w) 2-acetylaminofluorene was supplied in the diets. The control group had free access to water for 13 weeks (CTR13). Tea infusion was provided from the beginning of the experiment for 13 weeks (PRE13) or from the post-initiation stage until week 13 (POST13). Three other groups (CTR24, PRE24 and POST24) were added to examine the longer-term effects (24 weeks) with the same experimental design. The percentage area of liver sections that were positive for hepatic placental glutathione S-transferase (GST-P), which was used as a marker of preneoplastic lesions, was smaller in PRE13 (20.2 +/- 5.0%, mean +/- SD) and POST13 (26.0 +/- 4.8%) than in CTR13 (33.2 +/- 5.8%, p<0.05). Over the longer period, the GST-P lesions were significantly smaller for both PRE24 and POST24 (21.6 +/- 8.5% and 22.2 +/- 4.0%, respectively) than for CTR24 (28.6 +/- 5.1%, p<0.05), but there was no significant difference between PRE24 and POST24. The liver content of thiobarbituric acid reactive substances was significantly lower in the tea groups than in the controls (p<0.05). However, no significant differences were observed among groups of GST activity. The results show that tea consumption exhibits a stronger short-term initiation-inhibiting ability in liver carcinogenesis, but over a longer period, the preventive effects of green tea ingestion do not differ in post- and pre-initiation.
Keyword
MeSH Terms
-
2-Acetylaminofluorene
Animals
Diet
Diethylnitrosamine
Eating
Glutathione Transferase
Humans
Liver
Male
Rats
Rats, Sprague-Dawley
Research Design
Tea
Thiobarbiturates
Thiobarbituric Acid Reactive Substances
Water
2-Acetylaminofluorene
Diethylnitrosamine
Glutathione Transferase
Tea
Thiobarbiturates
Thiobarbituric Acid Reactive Substances
Water
Figure
Reference
-
1. Alagol H, Erdem E, Sancak B, Turkmen G, Camlibel M, Bugdayci G. Nitric oxide biosynthesis and malondiadehyde levels in advanced breast cancer. Aust N Z J Surg. 1999. 69:647–650.
Article2. Ahmad N, Katiyar SK, Mukhtar H. Antioxidants in chemoprevention of skin cancer. Curr Probl Dermatol. 2001. 29:128–139.
Article3. American Institute of Nutrition. Second report on the AIN Ad Hoc Committee on standards for nutritional studies. J Nutr. 1980. 110:1726.4. Arab L, Il'yasova D. The epidemiology of tea consumption and colorectal cancer incidence. J Nutr. 2003. 133:3310S–3318S.
Article5. Barret JR. Plants provide prevention. Environ Health Perspect. 2002. 110:180.6. Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978. 52:302–310.7. Chen C, Yu R, Owuor ED, Kong AN. Activation of antioxidant-response element (ARE), miotogen-activated protein kinases (MAPKs) and caspases by major green tea polyphenol components during cell survival and death. Arch Pharm Res. 2000. 23:605–612.
Article8. Conney AH, Lou YR, Xie JG, Osawa T, Newmark HL, Liu Y, Chang RL, Huang MT. Some perspectives on dietary inhibition of carcinogenesis: studies with curcumin and tea. Proc Soc Exp Biol Med. 1997. 216:234–245.
Article9. Dreosti IE. Bioactive ingredients: antioxidants and polyphenols in tea. Nutr Rev. 1996. 54:S51–S58.
Article10. Farombi EO, Tahnteng JG, Agboola AO, Nwankwo JO, Emerole GO. Chemoprevention of 2-acetylaminofluorene-induced hepatotoxicity and lipid peroxidation in rats by Kolaviron-A Garcinia kola Seed Extract. Food Chem Toxicol. 2000. 38:535–541.
Article11. Feng Q, Kumagai T, Torii Y, Nakamura Y, Osawa T, Uchida K. Anticarcinogenic antioxidants as inhibitors against intracellular oxidative stress. Free Radic Res. 2001. 35:779–788.
Article12. Gescher AJ, Sharma RA, Steward WP. Cancer chemoprevention by dietary constituents: a tale of failure and promise. Lancet Oncol. 2001. 2:371–379.
Article13. Guyton KZ, Kensler TW. Prevention of liver cancer. Curr Opin Oncol. 1997. 9:492–496.
Article14. Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferase. J Biol Chem. 1974. 249:7130–7139.15. Henning SM, Niu Y, Liu Y. Bioavailability and antioxidant effect of epigallocatechin gallate administered in purified form versus as green tea extract in healthy individuals. J Nutr Biochem. 2005. 16:610–616.
Article16. Huang H, Xu X. Anticancer activity of tea: evidence from recent animal experiments and human studies. Journal of Tea Science. 2004. 24:1–11.17. Ke L, Yu P, Zhang ZX, Huang SS, Huang G, Ma XH. Congou tea drinking and oesophageal cancer in South China. Br J Cancer. 2002. 86:346–347.
Article18. Korea National Statistical Office. 2007 Annual report on the cause of death statistics. 2008. Seoul. Republic of Korea: Korea National Statistical Office.19. Lambert JD, Yang CS. Mechanisms of cancer prevention by tea constituents. J Nutr. 2003. 133:3262S–3267S.
Article20. Lin JK. Cancer chemoprevention by tea polyphenols through modulating signal transduction pathways. Arch Pharm Res. 2002. 25:561–571.
Article21. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951. 193:265–275.
Article22. McKay DL, Blumberg JB. The role of tea in human health: An update. J Am Coll Nutr. 2002. 21:1–13.
Article23. Miyata M, Takano H, Guo LQ, Nagata K, Yamazoe Y. Grapefruit juice intake does not enhance but rather protects against aflatoxin B1-induced liver DNA damage through a reduction in hepatic CYP3A activity. Carcinogenesis. 2004. 25:203–209.
Article24. Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes. J Biol Chem. 1964. 239:2370–2378.
Article25. Ozdemirler G, Aykac G, Uysal M, Oz H. Liver lipid peroxidation and glutathione-related defence enzyme systems in mice treated with paracetamol. J Appl Toxicol. 1999. 14:297–299.
Article26. Sohn OS, Surace A, Fiala ES, Richie JP, Colosimo S, Zang E, Weisburger JH. Effect of green and black tea on hepatic xenobiotic metabolizing systems in the male F344 rat. Xenobiotica. 1994. 24:119–127.
Article27. Su LJ, Arab L. Tea consumption and the reduced risk of colon cancer-results from a national prospective cohor study. Public Health Nutr. 2002. 5:419–426.28. Takada M, Ku Y, Habara K. Inhibitory effect of epigallocatechin-3-gallate on growth and invasion in human biliary tract carcinoma cells. World J Surg. 2002. 26:683–686.
Article29. Tatematsu M, Mera Y, Inoue T, Satoh K, Sato K, Ito N. Stable phenotypic expression of glutathione S-transferase placental type and unstable phenotypic experssion of γ-glutamyltransferase in rat liver preneoplastic and neoplastic lesion. Carcinogenesis. 1988. 9:215–220.
Article30. Tian Q, Miller EG, Ahmad H, Tang L, Patil BS. Differential inhibition of human cancer cell proliferation by citrus limonoids. Nutr Cancer. 2001. 40:180–184.
Article31. Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. 2001. 21:381–406.
Article32. Yang CS, Maliakal P, Meng X. Inhibition of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. 2002. 42:25–54.
Article33. Yang G-Y, Liao J, Kim K, Yurkow EH, Yang CS. Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis. 1998. 19:611–616.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Green Tea Infusion on the Preneoplastic Lesions and Peroxidation in Rat Hepatocarcinogenesis
- Effects of Green Tea on Weight Gain, Plasma and Liver Lipids and Lipid Peroxidation in Pair Fed Rats
- Association Between Green Tea Consumption and Lung Cancer Risk
- Transactivation of peroxisome proliferator-activated receptor alpha by green tea extracts
- The Effects of Extracts from Green Tea, Guajava Leaves and Rose Petals on Allergic Rhinitis: A Randomized Double Blind Study