J Korean Soc Spine Surg.
2004 Mar;11(1):14-23. 10.4184/jkss.2004.11.1.14.
The Expression and Function of the Tumor Necrosis Factor Receptor I (TNFRI), TNFRII, and X-Linked Inhibitor of Apoptosis Genes after Spinal Cord Injury in Rats
- Affiliations
-
- 1Department of Orthopaedic Surgery, School of Medicine, Chungnam National University, Daejon, Korea. jyyang@cnuh.co.kr
- KMID: 2003187
- DOI: http://doi.org/10.4184/jkss.2004.11.1.14
Abstract
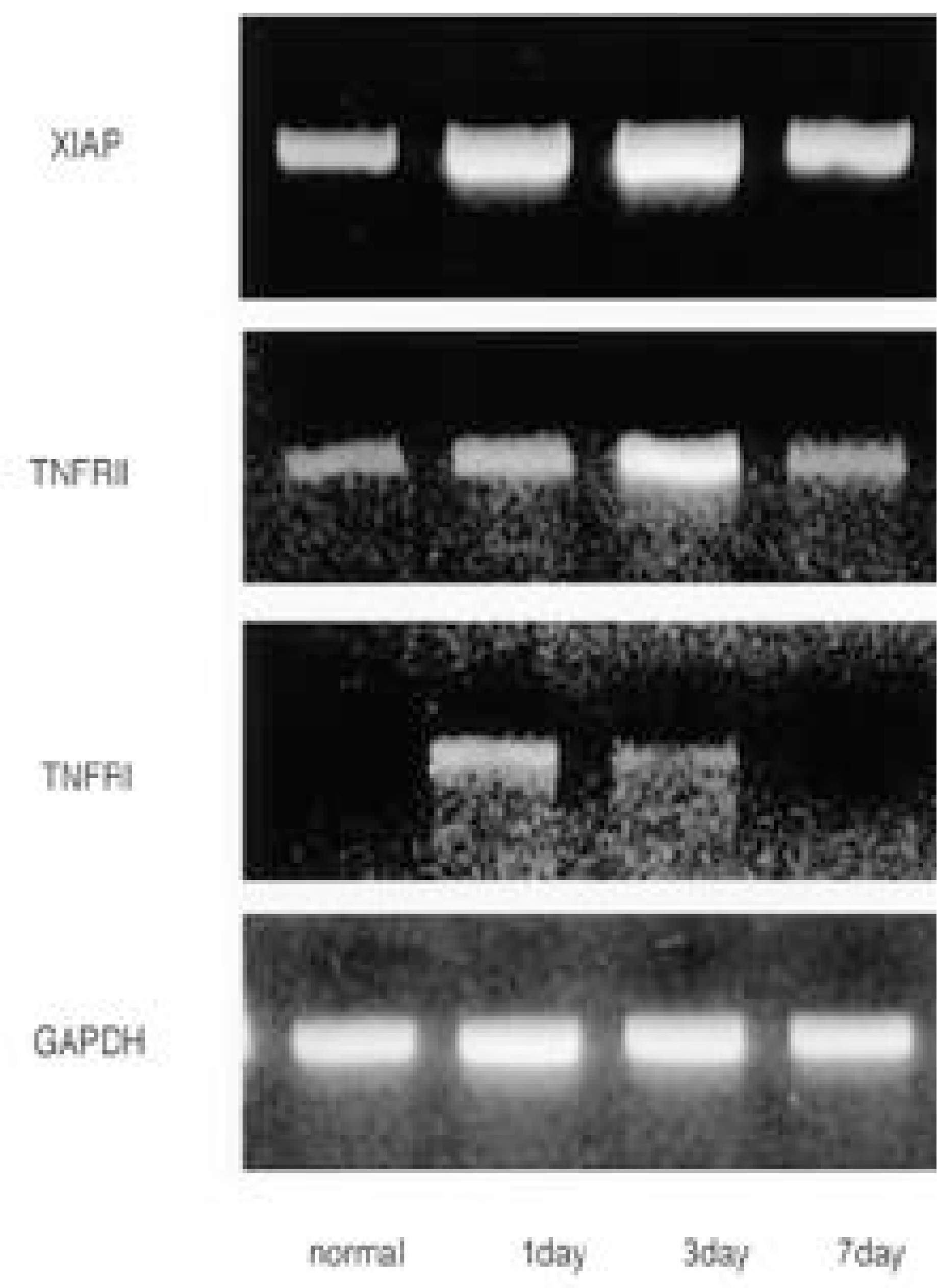
- STUDY DESIGN AND OBJECTIVE: Tumor necrosis factor-alpha(TNF-alpha), a key inflammatory mediator, has been demonstrated in spinal cord injury (SCI). However, the expression of TNF receptors following SCI remains to be identified. To elucidate the expressions of TNF receptor I (TNFRI), TNFRII, XIAP, and their function in SCI, in situ hybridization and RT-PCR were performed in a SCI model.
MATERIAL AND METHODS: Sprague-Dawley rats were anesthetized with halothane and laminectomized at the level of the eleventh and twelfth thoracic vertebra. Using a modified New York University Impactor, SCI was induced by dropping a 10 gm weight from a height of 20 mm. The rod of the impactor had a constant circular surface, 3 mm in diameter. After induction of the injury, rats were placed in a temperature and humidity-controlled chamber overnight.
RESULTS
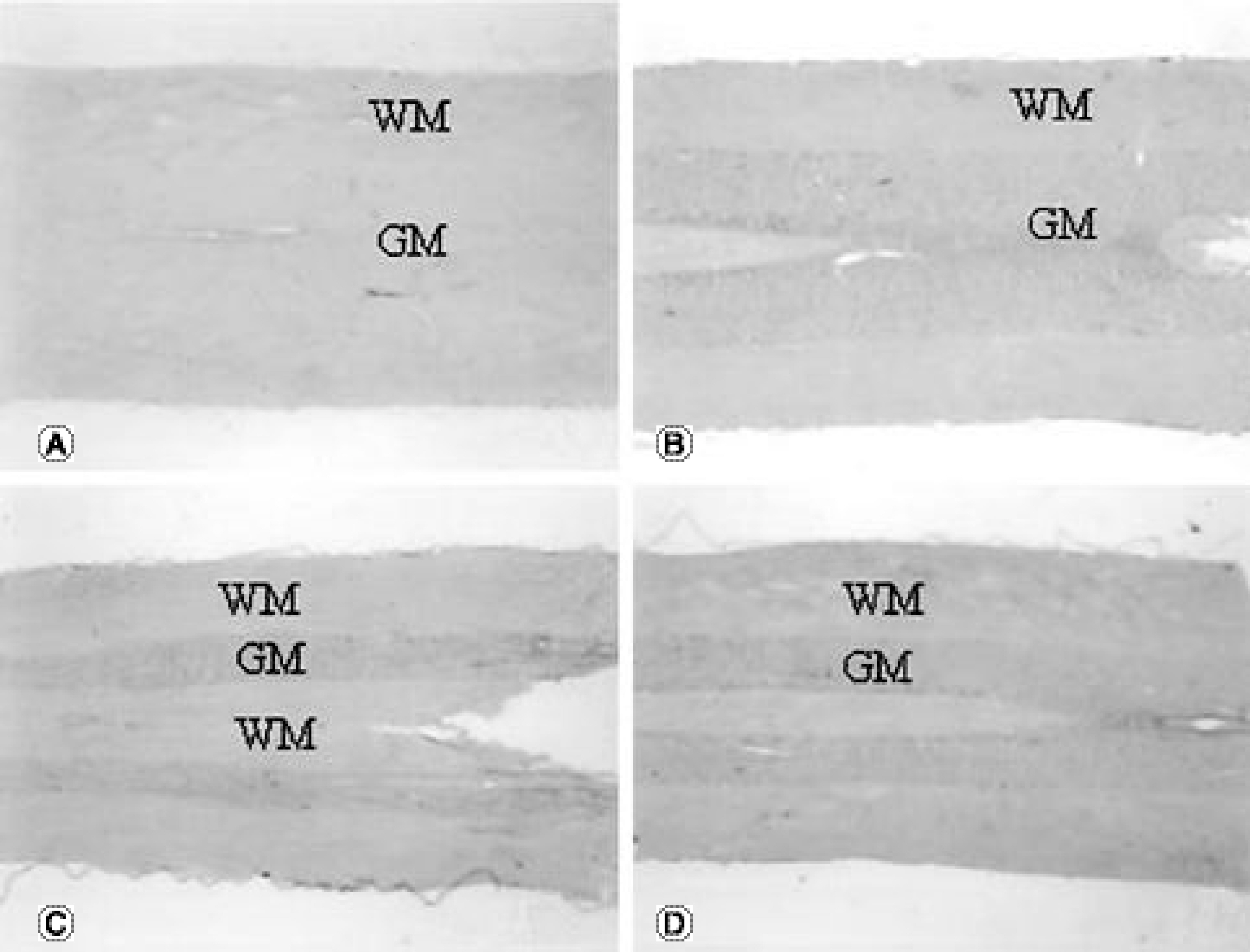
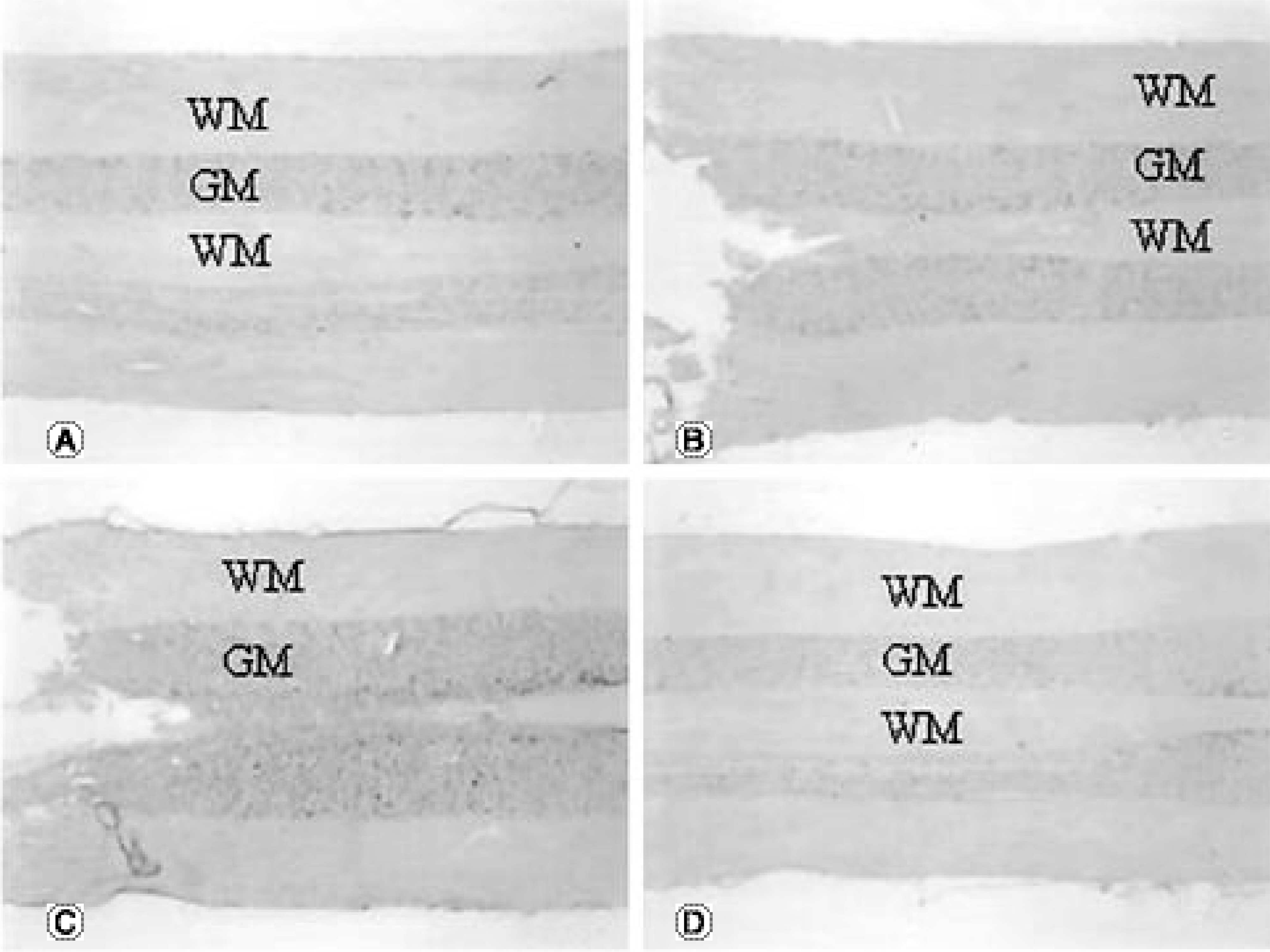
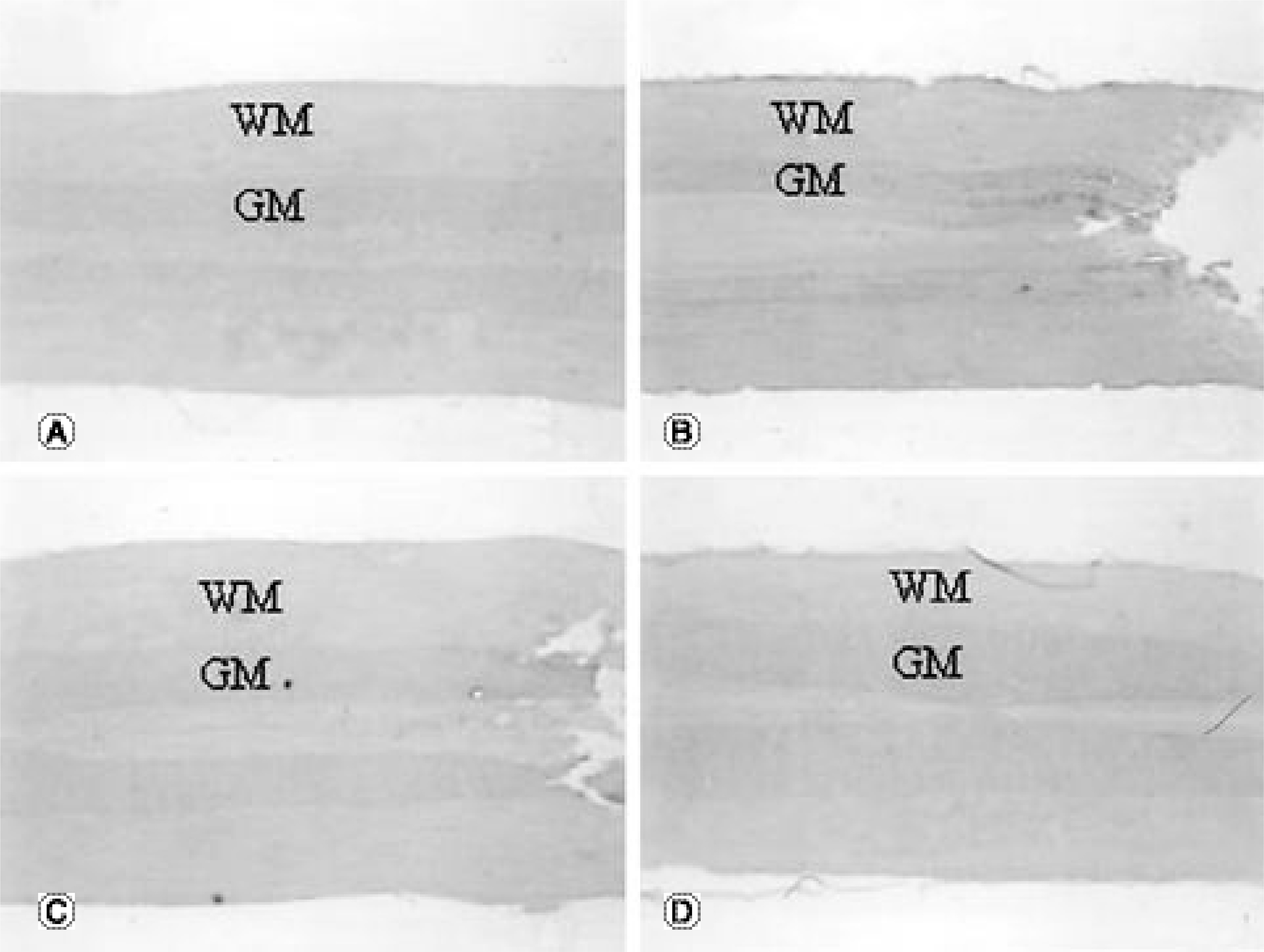
The TNFRI gene was not detected in the control rats, but the TNFRII gene was expressed in the neurons in the control rats. The TNFRI gene expression was maximally increased in the spinal cord 1 day after the SCI; however, that of the TNFRII gene occurred 3 days after the SCI. In the white matter, both the TNFRI and TNFRII genes were increased in the oligodendrocytes 3 days after the SCI. The XIAP gene was increased in neurons of the gray matter 1 and 3 days after the SCI, but was not detected in the white matter after the SCI.
CONCLUSION
Up-regulation of the expression of TNFRII occurs later than that of TNFRI in the spinal cord after a SCI. TNFR may be related to neuronal survival considering its similar expression pattern to that of XIAP. The results from these studies may lead to alternative therapeutic targets of TNF receptors in spinal cord injury, providing the basis for developing agonist and antagonist systems for TNF receptor subtypes and also for encouraging better strategies for the treatment of spinal cord disorders related to trauma.
Keyword
MeSH Terms
-
Animals
Apoptosis*
Gene Expression
Halothane
In Situ Hybridization
Necrosis
Neurons
Oligodendroglia
Rats*
Rats, Sprague-Dawley
Receptors, Tumor Necrosis Factor*
Spinal Cord Diseases
Spinal Cord Injuries*
Spinal Cord*
Spine
Tumor Necrosis Factor-alpha*
Up-Regulation
Halothane
Receptors, Tumor Necrosis Factor
Tumor Necrosis Factor-alpha
Figure
Cited by 3 articles
-
The Impact of Combined Treatments with Aminoguanidine and Methylprednisolone on Neurological Recovery after Spinal Cord Injury
Hyun-Ho Lee, Jun-Young Yang, June-Kyu Lee
J Korean Orthop Assoc. 2007;42(4):444-452. doi: 10.4055/jkoa.2007.42.4.444.The Effect of Methylprednisolone and Riluzole on Axonal Growth after Acute Spinal Cord Injury in Rats
Chang-Hwa Hong, Jun-Young Yang, June-Kyu Lee, Ho-Sup Song
J Korean Orthop Assoc. 2008;43(6):783-790. doi: 10.4055/jkoa.2008.43.6.783.Variations of Neurotrophic Factors and It's Importances in Spinal Cord Injured Rats and Beagle Dogs
In-Soo Song, Jun-Young Yang, June-Kyu Lee, Yong-Bum Joo, Soo-Min Cha
J Korean Soc Spine Surg. 2011;18(1):1-12. doi: 10.4184/jkss.2011.18.1.1.
Reference
-
1). Akassoglou K, Bauer J, Kassitotis G, et al. Oligoden -drocyte apoptosis and primary demyelination induced by local TNF/p55TNF receptor signaling in the central ner -vous system of transgenic mice: Models for multiple scle -rosis with primary oligodendrogliopathy. Am J Pathol. 1998; 153:801–813.2). Baker SJ, Reddy EP. Modulation of life and death by the TNF receptor superfamily. Oncogene. 1998; 17:3261–3270.
Article3). Bartholdi D, Schwab ME. Expression of pro-inflamatory cytokine and chemokine mRNA upon experimental spinal cord injury in mouse: an in situ hybridization study. Eur J Neurosci. 1997; 9:1422–1438.4). Brandoli C, Shi B, Pflug B, Andrews P, Wrathall JR, Mocchetti I. Dexamethasone reduces the expression of p75 neurotrophin receptor and apoptosis in contused spinal cord. Mol Brain Res. 2001; 87:61–70.
Article5). Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nature Med. 1997; 3:73–76.
Article6). Dsouza S, Alinauskas K, McCrea E, et al. Differential susceptibility of human CNS-derived cell populations to TNF-dependent and independent immune-mediated injury. J Neurosci. 1995; 15:7293–7300.7). Glazner GW, Mattson MP. Differential effects of BDNF, ADNF9, and TNF-alpha on levels of NMDA receptor sub -units, calcium homeostasis, and neuronal vulnerability to excitotoxicity. Exp Neurol. 2000; 161:442–452.8). Johnson Jr EM, Greenlund LJ, Akins PT, Hsu CY. Neuronal apoptosis: current understanding of molecular mechanisms and potential role in ischemic brain injury. J Neurotrauma. 1995; 12:843–852.9). Katoh K, Idkata T, Katoh S, et al. Induction and its spread of apoptosis in rat spinal cord after mechanical trauma. Neurosci Lett. 1996; 216:9–12.
Article10). Kerr JFR, Harmon BV. Definition and incidence of apoptosis: an historical perspective. Apoptosis: the molecular basis of cell death. Tomei LD, Cope Fo, editors. eds). 1991. p. p. 5. New York: Cold spring Harbor Laboratory.11). Kim GM, Xu J, Xu J, et al. Tumor necrosis factor receptor deletion reduces nuclear factor-kβ acti va tio n, cellular inhibitor of apoptosis protein 2 expression and functional recovery after traumatic spinal cord injury. J Neurosci. 2001; 21:6617–6625.12). Kraus JF, Franti CE, Riggins RS. Incidence of traumatic spinal cord lesions. J Chronic Dis. 1975; 28:471–92.
Article13). Kyriakis JM. Life-or-death decisions. Nature. 2001; 414:265–266.
Article14). Lee YB, Yune TY, Baik SY, et al. Role of tumor necrosis factor-alpha in neuronal and glial apoptosis after spinal cord injury. Exp Neurol. 2000; 166:190–195.15). Li G, Brodin LG, Farooque M, Funa K, Holtz A, Wang WL, Olsson Y. Apoptosis and expression of Bcl-2 after compression trauma to rat spinal cord. Exp Neurol. 1996; 55:280–289.
Article16). Liu J, Marino MW, Wong G, et al. TNF is a potent antiinflammatory cytokine in autoimmue-mediated demyelination. Nat Med. 1998; 4:78–83.17). Liu XZ, Xu XM, Hu R, Du C, Zhang SX, et al. Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci. 1997; 17:5395–5406.
Article18). Nawashiro H, Tasaki K, Ruetzler CA, Hallenbeck JM. TNF-alpha pretreatment induces protective effects against focal cerebral ischemia in mice. J Cereb Blood Flow Med -tab. 1997; 17:483–490.19). Papadopoulos SM. Spinal cord injury. Curr Opin Neurol Neurosurg. 1992; 55:554–557.20). Scherbel U, Raghupathi R, Nakamura M, et al. Differential acute and chronic responses of tumor necrosis factor-deficient mice to experimental brain injury. Proc Natl Acad Sci USA. 1999; 96:8721–8726.
Article21). Shuman SL, Bresnahan JC, Beattie MS. Apoptosis of microglia and oligodendrocytes after spinal cord contusion in rats. J Neurosci Res. 1997; 50:798–808.
Article22). Yang JY, Lee JK, Chung SY, Hong CH. The changes in expression of tumor necrosis factor receptor I after spinal cord injury. Euro. Spine. 2002; 11:415.23). Yang JY, Lee JK, Kim KC. A comparative study of behavioral and immunohistochemical changes after spinal cord injury between young and adult rats. Euro. Spine. 2002; 11:418.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Impact of Combined Treatments with Aminoguanidine andMethylprednisolone on Neurological Recovery after Spinal Cord Injury
- The Blocking of TNF-alpha by RNA Interference and Its Influence on Synovial Fibroblast and Chondrocytes
- Tumor Necrosis Factor-alpha and Apoptosis Following Spinal Nerve Ligation Injury in Rats
- The Apoptosis and Expression of p53, Bcl-2 in Graded Contusion Injury of Rat Spinal Cord
- Effects of Tumor Necrosis Factor Alpha Blocker Adalimumab in Experimental Spinal Cord Injury