Anat Cell Biol.
2011 Jun;44(2):85-97. 10.5115/acb.2011.44.2.85.
Expression of ciliary neurotrophic factor and its receptor in experimental obstructive nephropathy
- Affiliations
-
- 1Department of Anatomy, College of Medicine, The Catholic University of Korea, Seoul, Korea. jhcha@catholic.ac.kr
- KMID: 1447418
- DOI: http://doi.org/10.5115/acb.2011.44.2.85
Abstract
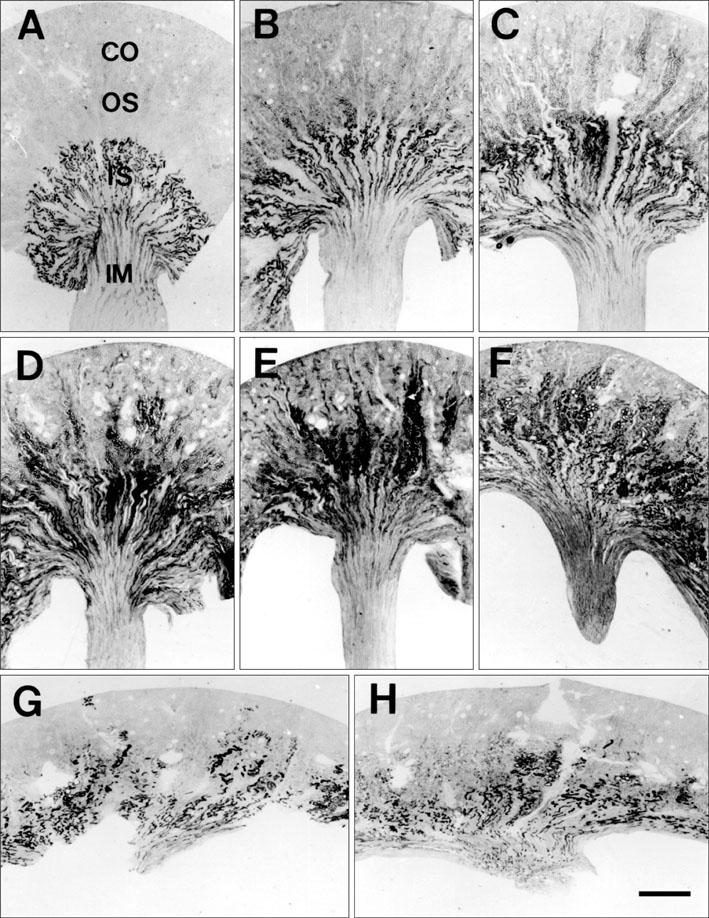
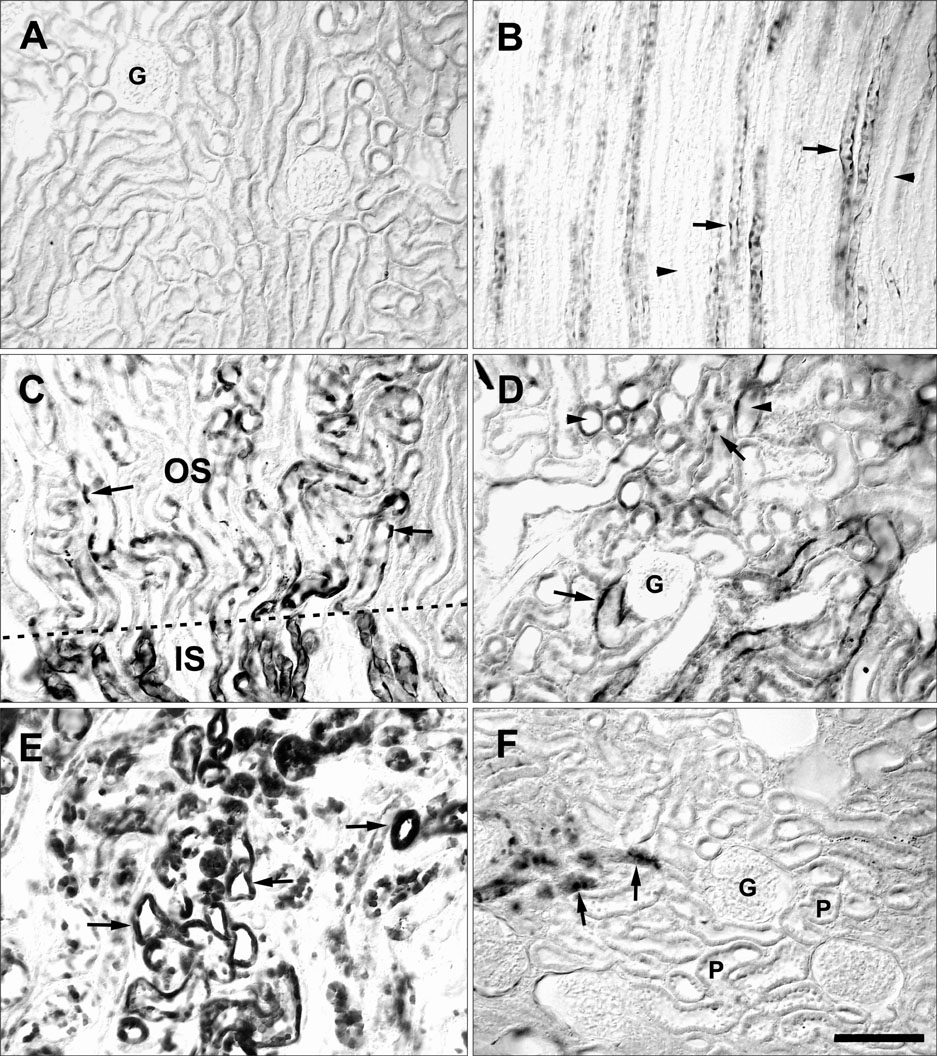
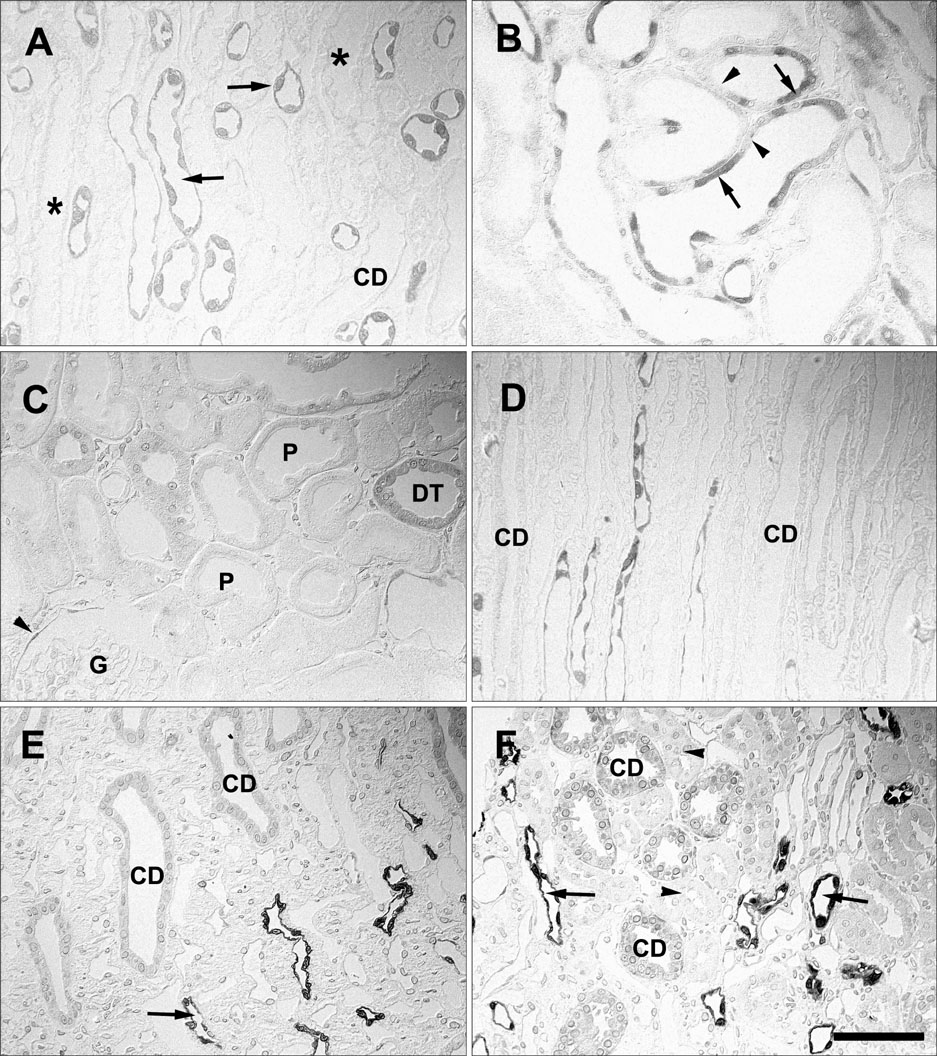
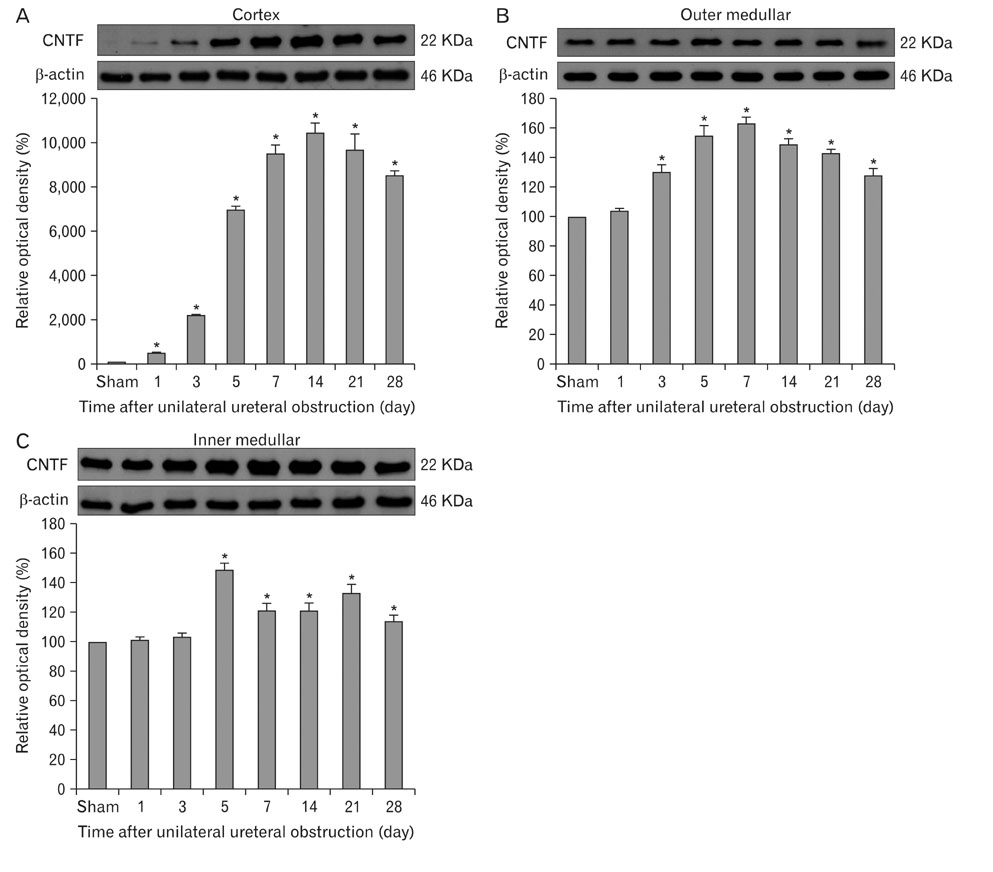
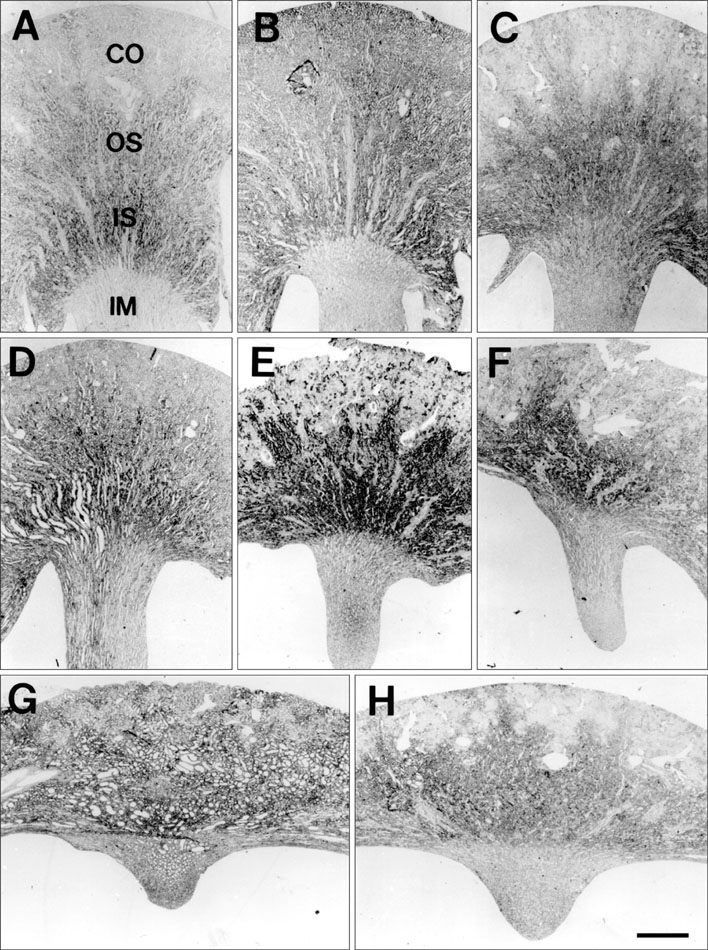
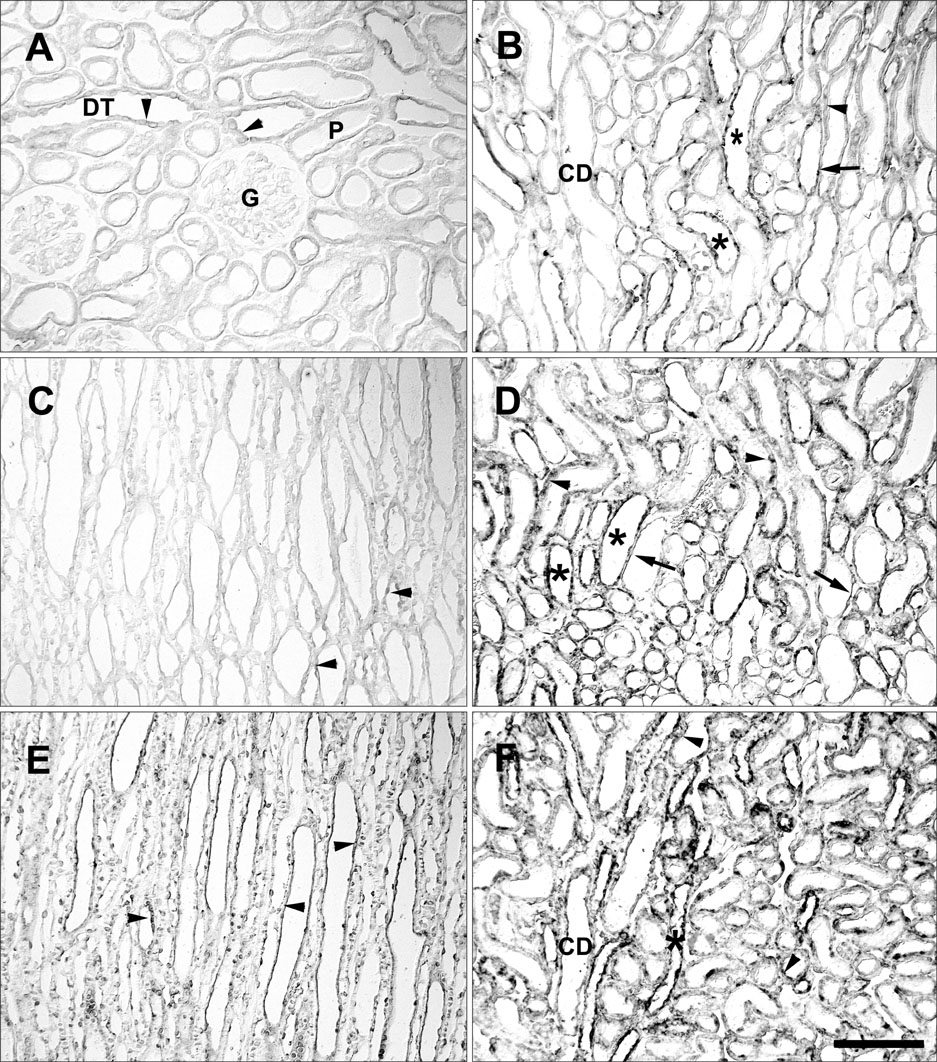
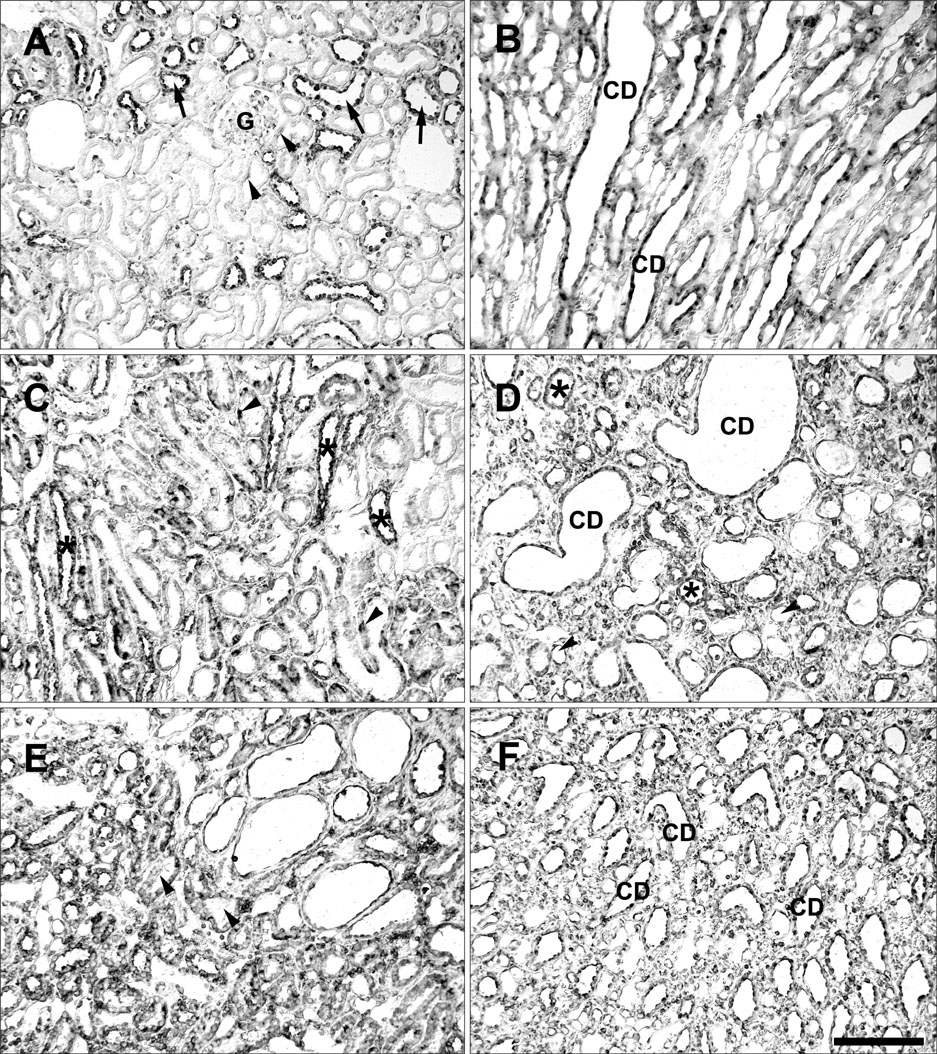
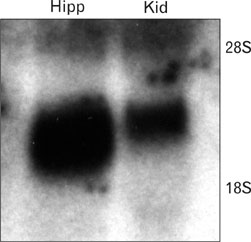
- Ciliary neurotrophic factor (CNTF) is well known as a growth/survival factor of neuronal tissue. We investigated the expression of CNTF and its specific receptor alpha (CNTFRalpha) in a unilateral ureteral obstruction (UUO) model. Complete UUO was produced by left ureteral ligation in Sprague-Dawley rats. The animals were sacrificed on days 1, 3, 5, 7, 14, 21, and 28 after UUO. The kidneys were fixed, and processed for both immunohistochemistry and in situ hybridization. CNTF immunoreactivity in sham-operated kidneys was observed only in the descending thin limb (DTL) of the loop of Henle. In UUO kidneys, CNTF expression was induced in the S3 segment (S3s) of the proximal tubule from day 1, and progressively expanded into the entire S3s and a part of the convoluted proximal tubules, distal tubules (DT), and glomerular parietal epithelium up to day 7. Upregulated CNTF expression was maintained to day 28. From day 14, the inner medullary collecting duct showed weak CNTF immunoreactivity. The CNTFRalpha mRNA hybridization signal in sham-operated kidneys was weakly detected in the DTL, DT, medullary thick ascending limb, and in a few S3s cells. After UUO, CNTFRalpha mRNA expression increased progressively in both the renal cortex and the medulla up to day 7 and increased expression was maintained until day 28. The results suggest that the S3s may be the principal induction site for CNTF in response to renal injury, and that CNTF may play a role in chronic renal injury.
Keyword
MeSH Terms
-
Animals
Chimera
Ciliary Neurotrophic Factor
Ciliary Neurotrophic Factor Receptor alpha Subunit
Epithelium
Extremities
Immunohistochemistry
In Situ Hybridization
Kidney
Ligation
Loop of Henle
Neurons
Rats, Sprague-Dawley
RNA, Messenger
Ureter
Ureteral Obstruction
Ciliary Neurotrophic Factor
Ciliary Neurotrophic Factor Receptor alpha Subunit
RNA, Messenger
Figure
Reference
-
1. Adler R, Landa KB, Manthorpe M, Varon S. Cholinergic neuronotrophic factors: intraocular distribution of trophic activity for ciliary neurons. Science. 1979. 204:1434–1436.2. Ip NY, Nye SH, Boulton TG, Davis S, Taga T, Li Y, Birren SJ, Yasukawa K, Kishimoto T, Anderson DJ, Stahl N, Yancopoulos GD. CNTF and LIF act on neuronal cells via shared signaling pathways that involve the IL-6 signal transducing receptor component gp130. Cell. 1992. 69:1121–1132.3. Ikeda K, Iwasaki Y, Shiojima T, Kinoshita M. Neuroprotective effect of various cytokines on developing spinal motoneurons following axotomy. J Neurol Sci. 1996. 135:109–113.4. Humes HD, Lake EW, Liu S. Renal tubule cell repair following acute renal injury. Miner Electrolyte Metab. 1995. 21:353–365.5. Heinrich PC, Behrmann I, Müller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998. 334(Pt 2):297–314.6. Davis S, Aldrich TH, Stahl N, Pan L, Taga T, Kishimoto T, Ip NY, Yancopoulos GD. LIFR beta and gp130 as heterodimerizing signal transducers of the tripartite CNTF receptor. Science. 1993. 260:1805–1808.7. Ohta K, Hara H, Hayashi K, Itoh N, Ohi T, Ohta M. Tissue expression of rat ciliary neurotrophic factor (CNTF) mRNA and production of the recombinant CNTF. Biochem Mol Biol Int. 1995. 35:283–290.8. Ohta M, Ohi T, Nishimura M, Itoh N, Hayashi K, Ohta K. Distribution of and age-related changes in ciliary neurotrophic factor protein in rat tissues. Biochem Mol Biol Int. 1996. 40:671–678.9. Ong AC, Fine LG. Tubular-derived growth factors and cytokines in the pathogenesis of tubulointerstitial fibrosis: implications for human renal disease progression. Am J Kidney Dis. 1994. 23:205–209.10. Yang CW, Lim SW, Han KW, Ahn HJ, Park JH, Kim YH, Kirsh M, Cha JH, Park JH, Kim YS, Kim J, Bang BK. Upregulation of ciliary neurotrophic factor (CNTF) and CNTF receptor alpha in rat kidney with ischemia-reperfusion injury. J Am Soc Nephrol. 2001. 12:749–757.11. Kim J, Kim YH, Cha JH, Tisher CC, Madsen KM. Intercalated cell subtypes in connecting tubule and cortical collecting duct of rat and mouse. J Am Soc Nephrol. 1999. 10:1–12.12. Davis S, Aldrich TH, Valenzuela DM, Wong VV, Furth ME, Squinto SP, Yancopoulos GD. The receptor of ciliary neurotrophic factor. Science. 1991. 253:59–63.13. Truong LD, Petrusevska G, Yang G, Gurpinar T, Shappell S, Lechago J, Rouse D, Suki WN. Cell apoptosis and proliferation in experimental chronic obstructive uropathy. Kidney Int. 1996. 50:200–207.14. Nguyen HT, Wu HY, Baskin LS, Kogan BA. High urinary flow accelerates renal injury in young rats with partial unilateral ureteral obstruction. J Urol. 2000. 163:1904–1907.15. Adler R. Ciliary neurotrophic factor as an injury factor. Curr Opin Neurobiol. 1993. 3:785–789.16. Sleeman MW, Anderson KD, Lambert PD, Yancopoulos GD, Wiegand SJ. The ciliary neurotrophic factor and its receptor, CNTFR alpha. Pharm Acta Helv. 2000. 74:265–272.17. Stöckli KA, Lottspeich F, Sendtner M, Masiakowski P, Carroll P, Götz R, Lindholm D, Thoenen H. Molecular cloning, expression and regional distribution of rat ciliary neurotrophic factor. Nature. 1989. 342:920–923.18. Lillien LE, Sendtner M, Rohrer H, Hughes SM, Raff MC. Type-2 astrocyte development in rat brain cultures is initiated by a CNTF-like protein produced by type-1 astrocytes. Neuron. 1988. 1:485–494.19. Lin LF, Mismer D, Lile JD, Armes LG, Butler ET 3rd, Vannice JL, Collins F. Purification, cloning, and expression of ciliary neurotrophic factor (CNTF). Science. 1989. 246:1023–1025.20. Kamiguchi H, Yoshida K, Sagoh M, Sasaki H, Inaba M, Wakamoto H, Otani M, Toya S. Release of ciliary neurotrophic factor from cultured astrocytes and its modulation by cytokines. Neurochem Res. 1995. 20:1187–1193.21. Lee MY, Naumann T, Kirsch M, Frotscher M, Hofmann HD. Transient up-regulation of ciliary neurotrophic factor receptor-alpha mRNA in axotomized rat septal neurons. Eur J Neurosci. 1997. 9:622–626.22. Chevalier RL, Goyal S, Wolstenholme JT, Thornhill BA. Obstructive nephropathy in the neonatal rat is attenuated by epidermal growth factor. Kidney Int. 1998. 54:38–47.23. Chevalier RL, Goyal S, Kim A, Chang AY, Landau D, LeRoith D. Renal tubulointerstitial injury from ureteral obstruction in the neonatal rat is attenuated by IGF-1. Kidney Int. 2000. 57:882–890.24. Truong LD, Gaber L, Eknoyan G. Obstructive uropathy. Contrib Nephrol. 2011. 169:311–326.25. Elbjeirami WM, Truong LD, Tawil A, Wang W, Dawson S, Lan HY, Zhang P, Garcia GE, Wayne Smith C. Early differential expression of oncostatin M in obstructive nephropathy. J Interferon Cytokine Res. 2010. 30:513–523.26. Akin M, Demirbilek S, Ay S, Gurunluoglu K, Turkmen E, Tas E, Aksoy RT, Baykarabulut A, Edali MN. Attenuation of ureteral obstruction-induced renal injury by polyenyl phos phatidylcholine. Int J Urol. 2007. 14:350–356.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Polymorphism of the Ciliary Neurotrophic Factor (CNTF) Gene in Korean
- microRNA-146a Promotes Growth of Acute Leukemia Cells by Downregulating Ciliary Neurotrophic Factor Receptor and Activating JAK2/STAT3 Signaling
- Delayed Treatment of Capsaicin Produces Partial Motor Recovery by Enhancing Dopamine Function in MPPâº-lesioned Rats via Ciliary Neurotrophic Factor
- The Effect of Recombinant Tyrosine Hydroxylase Expression on the Neurogenic Differentiation Potency of Mesenchymal Stem Cells
- Neuronal Rescue by Neurotrophic Factors in Human Fetal Cerebral Neuron Cultures Exposed to Oxygen Radical Injury