J Vet Sci.
2011 Jun;12(2):187-189. 10.4142/jvs.2011.12.2.187.
Maturation of bone marrow-derived dendritic cells by a novel beta-glucan purified from Paenibacillus polymyxa JB115
- Affiliations
-
- 1College of Veterinary Medicine, Jeju National University, Jeju 690-756, Korea. jooh@jejunu.ac.kr
- 2College of Veterinary Medicine, Kyungpook National University, Daegu 702-701, Korea.
- 3College of Veterinary Medicine, Kangwon National University, Chuncheon 200-701, Korea.
- KMID: 1106562
- DOI: http://doi.org/10.4142/jvs.2011.12.2.187
Abstract
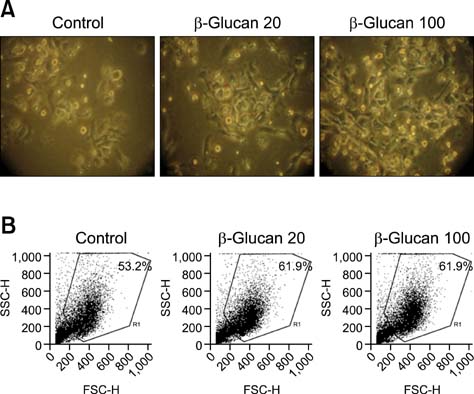
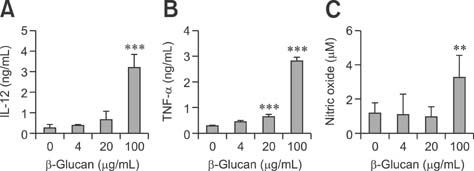
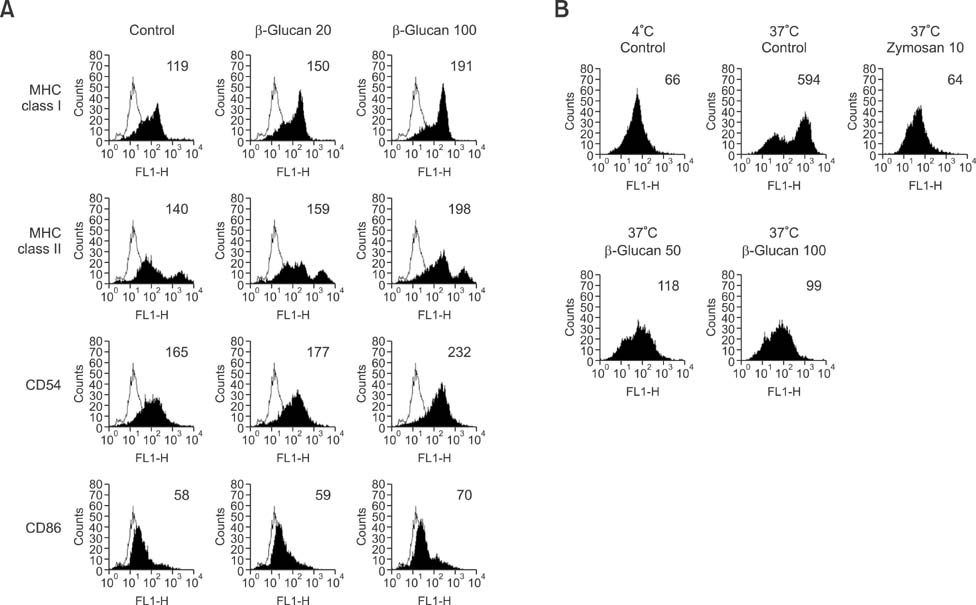
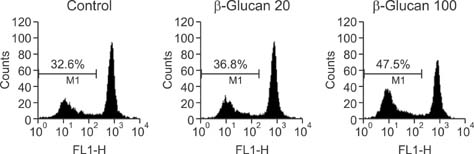
- We investigated the immunostimulatory effects of a novel beta-glucan purified from Paenibacillus (P.) polymyxa JB115 on bone marrow-derived dendritic cells (DCs), a type of potent antigen-presenting cells. beta-glucan isolated from P. polymyxa JB115 enhanced the viability and induced the maturation of DCs. beta-glucan markedly increased the cytokine production of DCs and surface expression of DC markers. In addition, DCs treated with beta-glucan showed a higher capacity to stimulate allogeneic spleen cell proliferation compared to those treated with medium alone. These results demonstrate the effect of beta-glucan on DC maturation and may increase the use of beta-glucan.
Keyword
MeSH Terms
-
Animals
Bone Marrow Cells/cytology/*drug effects/*immunology
Cell Survival/drug effects/*immunology
Dendritic Cells/cytology/*drug effects/*immunology
Flow Cytometry
Immunophenotyping/methods
Interleukin-12/analysis/immunology
Mice
Mice, Inbred BALB C
Nitric Oxide/analysis/immunology
Paenibacillus/*chemistry
Tumor Necrosis Factor-alpha/analysis/immunology
beta-Glucans/isolation & purification/*pharmacology
Figure
Cited by 1 articles
-
Distinct Effects of Monophosphoryl Lipid A, Oligodeoxynucleotide CpG, and Combination Adjuvants on Modulating Innate and Adaptive Immune Responses to Influenza Vaccination
Eun-Ju Ko, Young-Tae Lee, Youri Lee, Ki-Hye Kim, Sang-Moo Kang
Immune Netw. 2017;17(5):326-342. doi: 10.4110/in.2017.17.5.326.
Reference
-
1. Brown GD, Gordon S. Immune recognition of fungal β-glucans. Cell Microbiol. 2005. 7:471–479.
Article2. Chang ZQ, Lee JS, Gebru E, Hong JH, Jung HK, Jo WS, Park SC. Mechanism of macrophage activation induced by β-glucan produced from Paenibacillus polymyxa JB115. Biochem Biophys Res Commun. 2010. 391:1358–1362.
Article3. Esche C, Shurin GV, Kirkwood JM, Wang GQ, Rabinowich H, Pirtskhalaishvili G, Shurin MR. Tumor necrosis factor-α-promoted expression of Bcl-2 and inhibition of mitochondrial cytochrome c release mediate resistance of mature dendritic cells to melanoma-induced apoptosis. Clin Cancer Res. 2001. 7:3 Suppl. 974s–979s.4. Goodridge HS, Wolf AJ, Underhill DM. β-glucan recognition by the innate immune system. Immunol Rev. 2009. 230:38–50.
Article5. Heufler C, Koch F, Stanzl U, Topar G, Wysocka M, Trinchieri G, Enk A, Steinman RM, Romani N, Schuler G. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-γ production by T helper 1 cells. Eur J Immunol. 1996. 26:659–668.
Article6. Inaba K, Witmer-Pack M, Inaba M, Hathcock KS, Sakuta H, Azuma M, Yagita H, Okumura K, Linsley PS, Ikehara S, Muramatsu S, Hodes RJ, Steinman RM. The tissue distribution of the B7-2 costimulator in mice: abundant expression on dendritic cells in situ and during maturation in vitro. J Exp Med. 1994. 180:1849–1860.
Article7. Joo HG, Goedegebuure PS, Sadanaga N, Nagoshi M, von Bernstorff W, Eberlein TJ. Expression and function of galectin-3, a β-galactoside-binding protein in activated T lymphocytes. J Leukoc Biol. 2001. 69:555–564.8. Jung HK, Hong JH, Park SC, Park BK, Nam DH, Kim SD. Production and physicochemical characterization of β-glucan produced by Paenibacillus polymyxa JB115. Biotechnol Bioprocess Eng. 2007. 12:713–719.
Article9. Kim MH, Byon YY, Ko EJ, Song JY, Yun YS, Shin T, Joo HG. Immunomodulatory activity of ginsan, a polysaccharide of Panax ginseng, on dendritic cells. Korean J Physiol Pharmacol. 2009. 13:169–173.
Article10. Kim MH, Joo HG. Immunostimulatory effects of fucoidan on bone marrow-derived dendritic cells. Immunol Lett. 2008. 115:138–143.
Article11. Larsen CP, Ritchie SC, Hendrix R, Linsley PS, Hathcock KS, Hodes RJ, Lowry RP, Pearson TC. Regulation of immunostimulatory function and costimulatory molecule (B7-1 and B7-2) expression on murine dendritic cells. J Immunol. 1994. 152:5208–5219.12. Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001. 106:255–258.13. Steinman RM, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 1997. 156:25–37.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunostimulatory Effects of beta-glucan Purified from Paenibacillus polymyxa JB115 on Mouse Splenocytes
- A novel beta-glucan produced by Paenibacillus polymyxa JB115 induces nitric oxide production in RAW264.7 macrophages
- Defects in the differentiation and function of bone marrow-derived dendritic cells in non-obese diabetic mice
- Immunomodulatory Effects of Eckol, a Pure Compound of Ecklonia Cava, on Dendritic Cells
- Mycobacterium tuberculosis ESAT6 Drives the Activation and Maturation of Bone Marrow-Derived Dendritic Cells via TLR4-Mediated Signaling