Korean J Radiol.
2010 Jun;11(3):278-285. 10.3348/kjr.2010.11.3.278.
Time-Course Analysis of the Neuroanatomical Correlates of Sexual Arousal Evoked by Erotic Video Stimuli in Healthy Males
- Affiliations
-
- 1Department of Biomedical Engineering, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju 501-757, Korea. gwjeong@jnu.ac.kr
- 2Department of Radiology, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju 501-757, Korea.
- KMID: 946267
- DOI: http://doi.org/10.3348/kjr.2010.11.3.278
Abstract
OBJECTIVE
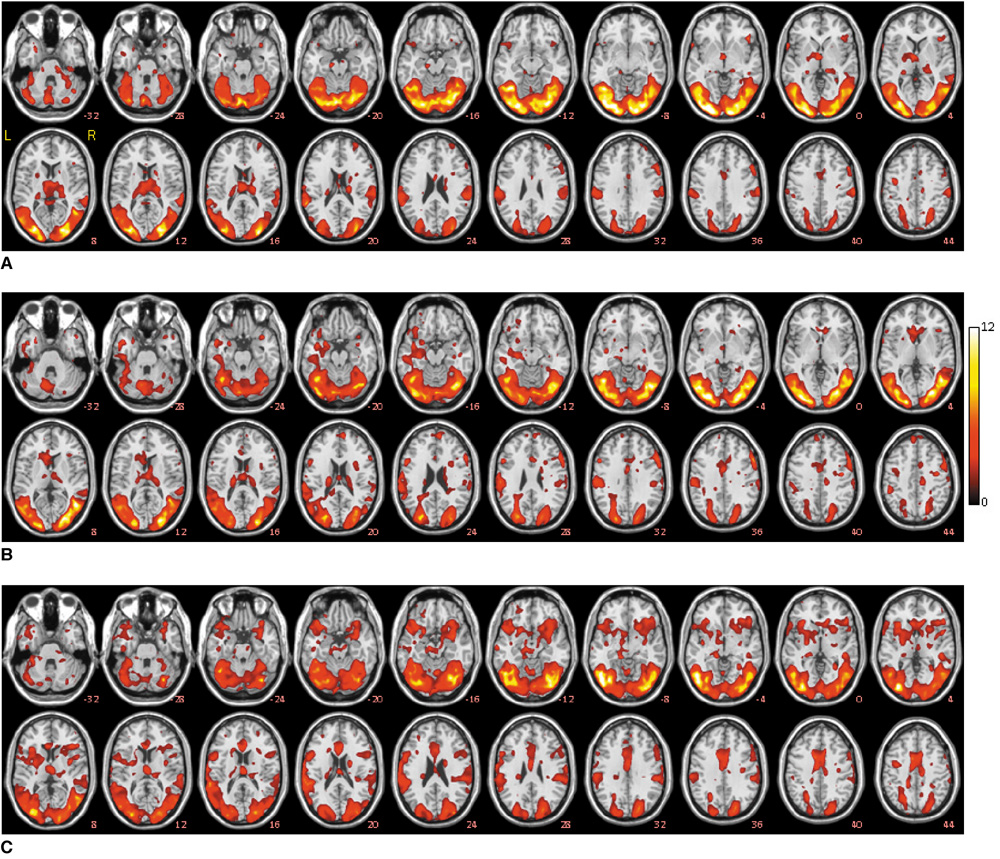
To assess the dynamic activations of the key brain areas associated with the time-course of the sexual arousal evoked by visual sexual stimuli in healthy male subjects. MATERIALS AND METHODS: Fourteen right-handed heterosexual male volunteers participated in this study. Alternatively combined rest period and erotic video visual stimulation were used according to the standard block design. In order to illustrate and quantify the spatiotemporal activation patterns of the key brain regions, the activation period was divided into three different stages as the EARLY, MID and LATE stages. RESULTS: For the group result (p < 0.05), when comparing the MID stage with the EARLY stage, a significant increase of the brain activation was observed in the areas that included the inferior frontal gyrus, the supplementary motor area, the hippocampus, the head of the caudate nucleus, the midbrain, the superior occipital gyrus and the fusiform gyrus. At the same time, when comparing the EARLY stage with the MID stage, the putamen, the globus pallidus, the pons, the thalamus, the hypothalamus, the lingual gyrus and the cuneus yielded significantly increased activations. When comparing the LATE stage with the MID stage, all the above mentioned brain regions showed elevated activations except the hippocampus. CONCLUSION: Our results illustrate the spatiotemporal activation patterns of the key brain regions across the three stages of visual sexual arousal.
MeSH Terms
Figure
Reference
-
1. Levin R, Riley A. The physiology of human sexual function. Psychiatry. 2007. 6:90–94.2. Schober JM, Pfaff D. The neurophysiology of sexual arousal. Best Pract Res Clin Endocrinol Metab. 2007. 21:445–461.3. Stoléru S, Grégoire MC, Gérard D, Decety J, Lafarge E, Cinotti L, et al. Neuroanatomical correlates of visually evoked sexual arousal in human males. Arch Sex Behav. 1999. 28:1–21.4. Redouté J, Stoléru S, Grégoire MC, Costes N, Cinotti L, Lavenne F, et al. Brain processing of visual sexual stimuli in human males. Hum Brain Mapp. 2000. 11:162–177.5. Graziottin A. Sexual arousal: similarities and differences between men and women. J Mens Health Gend. 2004. 1:215–223.6. Redouté J, Stoléru S, Pugeat M, Costes N, Lavenne F, Le Bars D, et al. Brain processing of visual sexual stimuli in treated and untreated hypogonadal patients. Psychoneuroendocrinology. 2005. 30:461–482.7. Stoléru S, Redouté J, Costes N, Lavenne F, Bars DL, Dechaud H, et al. Brain processing of visual sexual stimuli in men with hypoactive sexual desire disorder. Psychiatry Res. 2003. 124:67–86.8. Bocher M, Chisin R, Parag Y, Freedman N, Meir Weil Y, Lester H, et al. Cerebral activation associated with sexual arousal in response to a pornographic clip: a 15O-H2O PET study in heterosexual men. Neuroimage. 2001. 14:105–117.9. Arnow BA, Desmond JE, Banner LL, Glover GH, Solomon A, Polan ML, et al. Brain activation and sexual arousal in healthy, heterosexual males. Brain. 2002. 125:1014–1023.10. Ferretti A, Caulo M, Del Gratta C, Di Matteo R, Merla A, Montorsi F, et al. Dynamics of male sexual arousal: distinct components of brain activation revealed by fMRI. Neuroimage. 2005. 26:1086–1096.11. Moulier V, Mouras H, Pélégrini-Issac M, Glutron D, Rouxel R, Grandjean B, et al. Neuroanatomical correlates of penile erection evoked by photographic stimuli in human males. Neuroimage. 2006. 33:689–699.12. Park K, Seo JJ, Kang HK, Ryu SB, Kim HJ, Jeong GW. A new potential of blood oxygenation level dependent (BOLD) functional MRI for evaluating cerebral centers of penile erection. Int J Impot Res. 2001. 13:73–81.13. Karama S, Lecours AR, Leroux JM, Bourgouin P, Beaudoin G, Joubert S, et al. Areas of brain activation in males and females during viewing of erotic film excerpts. Hum Brain Mapp. 2002. 16:1–13.14. Mouras H, Stoléru S, Bittoun J, Glutron D, Pélégrini-Issac M, Paradis AL, et al. Brain processing of visual sexual stimuli in healthy men: a functional magnetic resonance imaging study. Neuroimage. 2003. 20:855–869.15. Hamann S, Herman RA, Nolan CL, Wallen K. Men and women differ in amygdala response to visual sexual stimuli. Nat Neurosci. 2004. 7:411–416.16. Yang JC, Jeong GW, Lee MS, Kang HK, Eun SJ, Kim YK, et al. Functional MR imaging of psychogenic amnesia: a case report. Korean J Radiol. 2005. 6:196–199.17. Yang JC. Functional neuroanatomy in depressed patients with sexual dysfunction: blood oxygenation level dependent functional MR imaging. Korean J Radiol. 2004. 5:87–95.18. Maravilla KR, Yang CC. Sex and the brain: the role of fMRI for assessment of sexual function and response. Int J Impot Res. 2007. 19:25–29.19. Friston KJ, Ashburner J, Frith CD, Poline J-B, Heather JD, Frackowiak RSJ. Spatial registration and normalisation of images. Hum Brain Mapp. 1995. 2:165–189.20. Friston KJ, Holmes AP, Poline J-B, Grasby PJ, Williams SCR, Frackowiak RSJ, et al. Analysis of fMRI time-series revisited. Neuroimage. 1995. 2:45–53.21. Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995. 2:189–210.22. Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996. 35:346–355.23. Hajnal JV, Myers R, Oatridge A, Schwieso JE, Young IR, Bydder GM. Artifacts due to stimulus correlated motion in functional imaging of the brain. Magn Reson Med. 1994. 31:283–291.24. Lee JM, Jeong GW, Kim HJ, Cho SH, Kang HK, Seo JJ, et al. Qualitative and quantitative measurement of human brain activity using pixel subtraction algorithm. J Korean Radiol Soc. 2004. 51:165–177. [Korean].25. Janssen E, Everaerd W, Spiering M, Janssen J. Automatic processes and the appraisal of sexual stimuli: toward an information processing model of sexual arousal. J Sex Res. 2000. 37:8–23.26. Kapp B, Cain M. Smelser N, Baltes P, editors. The neural basis of arousal. The international encyclopedia of social and behavioral sciences. 2001. Oxford: Elsevier Science Ltd;1463–1466.27. Giuliano F, Allard J. Dopamine and sexual function. Int J Impot Res. 2001. 13:S18–S28.28. MacLean PD, Ploog DW. Cerebral presentation of penile erection. J Neurophysiol. 1962. 25:29–55.29. MacLean PD, Denniston RH, Dua S. Further studies on cerebral representation of penile erection: caudal thalamus, midbrain, and pons. J Neurophysiol. 1963. 26:273–293.30. Michael RP, Clancy AN, Zumpe D. Effects of mating on c-fos expression in the brains of male macaques. Physiol Behav. 1999. 66:591–597.31. Ferris CF, Snowdon CT, King JA, Sullivan JM Jr, Ziegler TE, Olson DP, et al. Activation of neural pathways associated with sexual arousal in non-human primates. J Magn Reson Imaging. 2004. 19:168–175.32. Perachio AA, Marr LD, Alexander M. Sexual behavior in male rhesus monkeys elicited by electrical stimulation of preoptic and hypothalamic areas. Brain Res. 1979. 177:127–144.33. Robinson BW, Mishkin M. Penile erection evoked from forebrain structures in Macaca mulatta. Arch Neurol. 1968. 19:184–198.34. Liu YC, Salamone JD, Sachs BD. Lesions in medial preoptic area and bed nucleus of stria terminalis: differential effects on copulatory behavior and noncontact erection in male rats. J Neurosci. 1997. 17:5245–5253.35. Georgiadis JR, Holstege G. Human brain activation during sexual stimulation of the penis. J Comp Neurol. 2005. 493:33–38.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Sertraline on Current-Source Distribution of the High Beta Frequency Band: Analysis of Electroencephalography under Audiovisual Erotic Stimuli in Healthy, Right-Handed Males
- Difference of Brain Activation by Visual Erotic Stimuli in Young and Middle-aged Healthy Males
- Cerebral Activation Associated with Visually Evoked Sexual Arousal in the Limbic System: Functional MR Imaging
- Functional Neuroanatomy in Depressed Patients with Sexual Dysfunction: Blood Oxygenation Level Dependent Functional MR Imaging
- Qualitative and Quantitative Measurement of Brain Activity Associated with Visual Sexual Arousal in Males and Females: 3.0 Tesla Functional MR Imaging