Korean J Radiol.
2001 Sep;2(3):164-170. 10.3348/kjr.2001.2.3.164.
Sclerotherapy of Peritoneal Inclusion Cysts: Preliminary Results in Seven Patients
- Affiliations
-
- 1Department of Radiology, Seoul National University College of Medicine, Seoul, Korea. kimsh@radcom.snu.ac.kr
- KMID: 754102
- DOI: http://doi.org/10.3348/kjr.2001.2.3.164
Abstract
OBJECTIVE
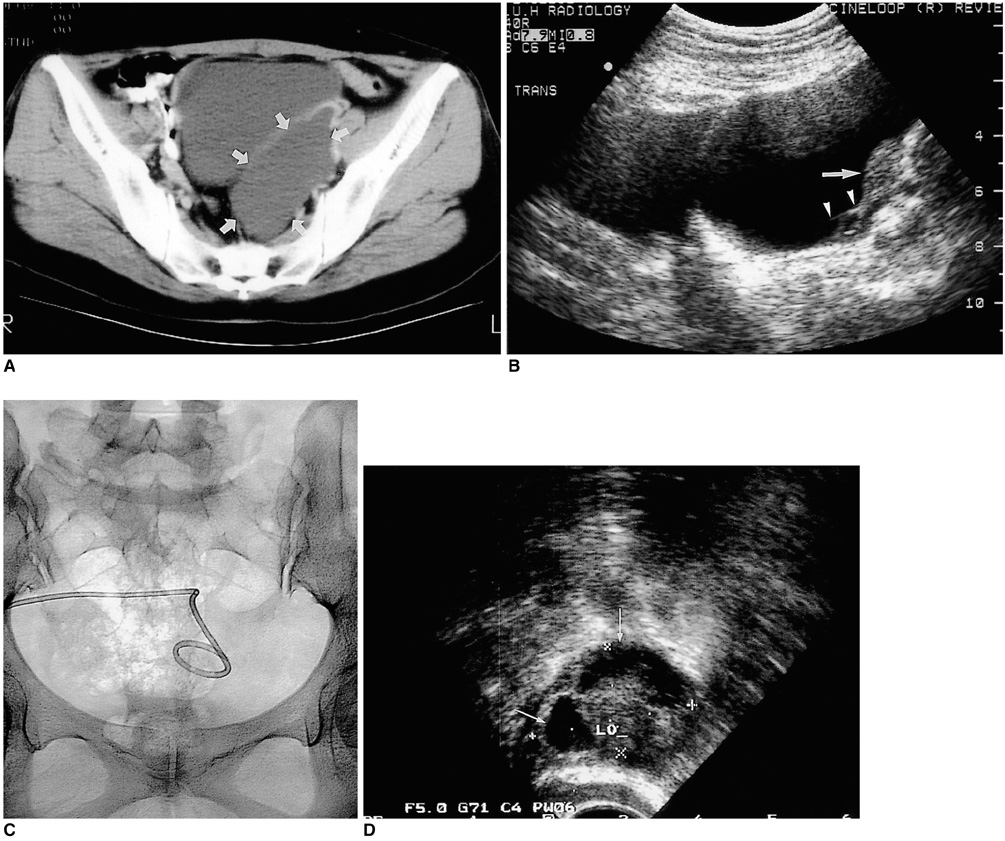
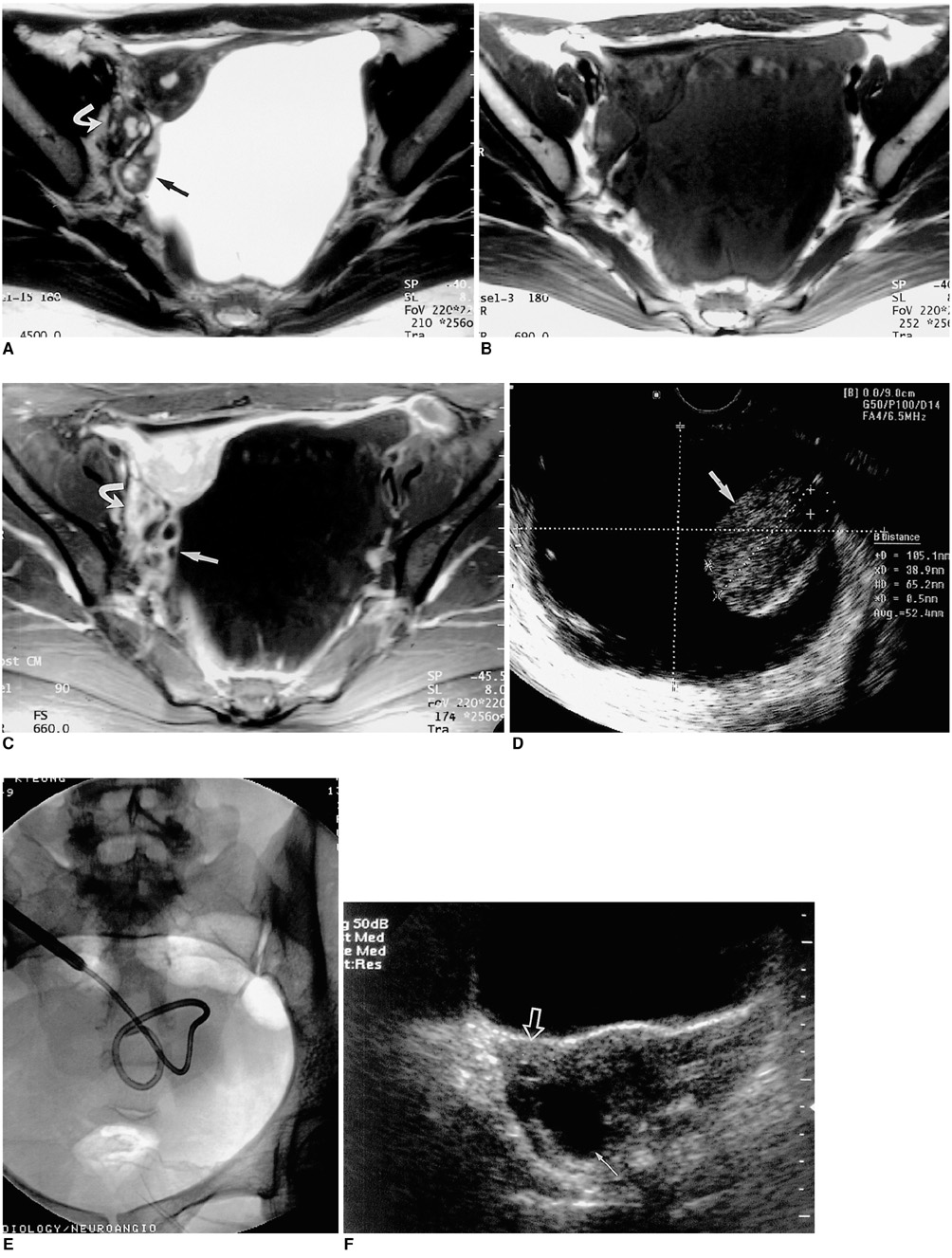
To evaluate the technical feasibility and the clinical effectiveness of sclerotherapy for the treatment of peritoneal inclusion cysts (PICs). MATERIALS AND METHODS: Between June 1996 and February 2001, eight PICs in seven female patients aged 28-43 (mean, 36) years were instilled with sclerosant (povidone-iodine in three, ethanol in three, both povidone-iodine and ethanol in one). All seven patients subsequently experienced less abdominal pain. After drainage via an 8.5-Fr pigtail catheter inserted in the PICs (transabdominally in six cases, transvaginally in one), sclerosant equivalent in volume to about one-third that of drained fluid was introduced daily until the drained volume was less than 5ml. Follow-up by means of clinical procedures and ultrasound was performed every three months, at which time the success rate, possible complications and recurrence were determined. RESULTS: Sclerotherapy was technically successful in all seven patients, though immediately after the procedure, minor complications were noted in three patients (mild pain in two, mild fever in one). During the follow-up of 4-60 (mean, 24.7) months, sclerotherapy proved successful and without long-term complications in all seven patients: lower abdominal pain disappeared and the diameter of the cysts decreased more than 50%, with complete regression in four cases. During the follow-up period there was no recurrence. CONCLUSION: Sclerotherapy following catheter insertion is technically feasible and effective for the treatment of PICs.
MeSH Terms
Figure
Reference
-
1. Jain KA. Imaging of peritoneal inclusion cysts. AJR. 2000. 174:1559–1563.2. Ross MJ, Welch WR, Scully RE. Multilocular peritoneal inclusion cysts (so-called cystic mesotheliomas). Cancer. 1989. 64:1336–1346.3. McFadden DE, Clement PB. Peritoneal inclusion cysts with mural mesothelial proliferation: A clinicopathological analysis of six cases. Am J Surg Pathol. 1986. 10:844–854.4. Schneider V, Partridge JR, Gutierrez F, Hurt WG, Maizels MS, Demay RM. Benign cystic mesothelioma involving the female genital tract: report of four cases. Am J Obstet Gynecol. 1983. 145:355–359.5. Lipitz S, Seidman DS, Schiff E, Achiron R, Menczer J. Treatment of pelvic peritoneal cysts by drainage and ethanol instillation. Obstet Gynecol. 1995. 86:297–299.6. Sohaey R, Gardner TL, Woodward PJ, Peterson CM. Sonographic diagnosis of peritoneal inclusion cysts. J Ultrasound Med. 1995. 14:913–917.7. Hoffer FA, Kozakewich H, Colodny A, Goldstein DP. Peritoneal inclusion cysts: ovarian fluid in peritoneal adhesions. Radiology. 1988. 169:189–191.8. Gussman D, Thickman D, Wheeler JE. Postoperative peritoneal cysts. Obstet Gynecol. 1986. 68(3):Suppl. 53S–55S.9. Kim JS, Lee HJ, Woo SK, Lee TS. Peritoneal inclusion cysts and their relationship to the ovaries: evaluation with sonography. Radiology. 1997. 204:481–484.10. Kurachi H, Murakami T, Maeda T, et al. Value of gonadotropin-releasing hormone agonist in diagnosing peritoneal pseudocysts. Acta Obstet Gynecol Scand. 1996. 75:294–297.11. Takeuchi K, Kitazawa S, Kitagaki S, Mauro T. Conservative management of post-operative peritoneal cysts associated with endometriosis. Int J Gynecol Obstet. 1998. 60:151–154.12. Rosai J. Rosai J. Peritoneum, retroperitoneum, and related structures. Ackerman's surgical pathology. 1996. St. Louis: Mosby;2137.13. Cancelmo RP. Sonographic demonstration of multilocular peritoneal inclusion cyst. J Clin Ultrasound. 1983. 11:334–335.14. Lees RF, Feldman PS, Brenbridge AN, Anderson WA, Buschi AJ. Inflammatory cysts of the pelvic periteum. AJR. 1978. 131:633–636.15. Kurachi H, Murakami T, Nakamura H, et al. Imaging of peritoneal psuedocysts: value of MR imaging compared with sonography and CT. AJR. 1993. 160:589–591.16. Hederstrom E, Forsberg L. Entrapped ovarian cyst-an unusual case of persistent abdominal pain. Acta Radiol. 1990. 31:285–286.17. Fleischer AC, Tait D, Mayo J, Burnett L, Simpson J. Sonographic features of ovarian remnants. J Ultrasound Med. 1998. 17:551–555.18. Komickx PR, Renaer M, Brosens IA. Origin of peritoneal fluid in women: an ovarian exudation product. Br J Obstet Gynaecol. 1980. 87:177–183.19. Hanna RM, Dahniya MH. Aspiration and sclerotherapy of symptomatic simple renal cysts: value of two injections of a sclerosing agent. AJR. 1996. 167:781–783.20. Simmonetti G, Profili S, Sergiacomi GL, Meloni GB, Orlacchio A. Percutaneous treatment of hepatic cysts by aspiration and sclerotherapy. Cardiovasc Intervent Radiol. 1993. 16:81–84.21. Troiano RN, Taylor KJ. Sonographically guided therapeutic aspiration of benign-appearing ovarian cysts and endometriomas. AJR. 1998. 171:1601–1605.22. Cho YS, Lee HK, Ahn IM, et al. Sonographically guided ethanol sclerotherapy for benign thyroid cysts: results in 22 patients. AJR. 2000. 174:213–216.23. Zuckerman DA, Yeager TD. Percutaneous ethanol sclerotherapy of postoperative lymphoceles. AJR. 1997. 169:433–437.24. Philip G, Reilly AL. Benign cystic mesothelioma. Br J Obstet Gynaecol. 1984. 91:932–938.25. Katsube Y, Mukai K, Silverberg SG. Cystic mesothelioma of the peritoneum: a report of five cases and a review of the literature. Cancer. 1982. 50:1615–1622.26. Miles JM, Hart WR, McMahon JT. Cystic mesothelioma of the peritoneum. Report of a case with multiple recurrences and a review of the literature. Cleve Clin Q. 1986. 53:109–114.27. Kairuloma M, Leinonen A, Stanlberg M, Paivansalo M, Kiviniemi H, Siniluoto T. Percutaneous aspiration and sclerotherapy for symptomatic hepatic cysts. Ann Surg. 1989. 210:208–215.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Clinical Study of Peritoneal Inclusion Cysts
- Peritoneal Inclusion Cysts: Changes on Follow-up Ultrasonography
- A Case of Peritoneal Inclusion Cyst caused by Chlamydia Trachomatis Infection
- Simple Hepatic Cysts Mimicking Biliary Cystic Neoplasm after Ultrasound-Guided Percutaneous Alcohol Sclerotherapy
- A Clinical analysis of sonographic and operation findings of peritoneal inclusion cyst