Yonsei Med J.
2008 Jun;49(3):366-371. 10.3349/ymj.2008.49.3.366.
The Role of CD4(+)CD25(bright) Regulatory T Cells in the Maintenance of Pregnancy, Premature Rupture of Membranes, and Labor
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Korea University College of Medicine, Seoul, Korea. haijkim@korea.ac.kr
- 2Department of Diagnostic Laboratory Medicine, Korea University College of Medicine, Seoul, Korea.
- KMID: 724250
- DOI: http://doi.org/10.3349/ymj.2008.49.3.366
Abstract
- PURPOSE
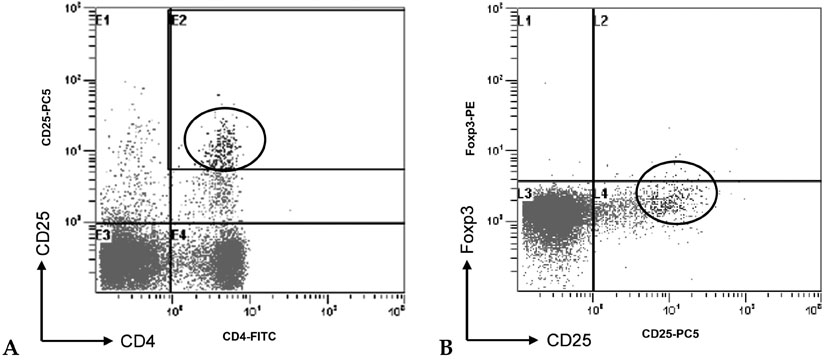
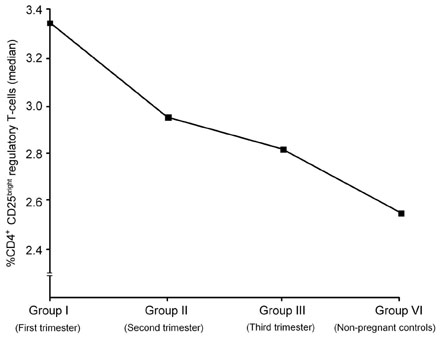
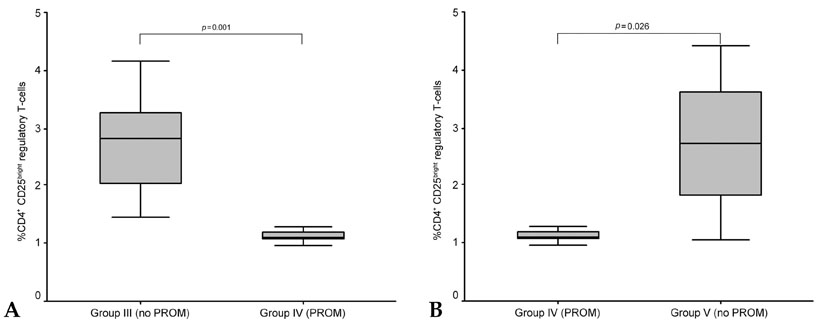
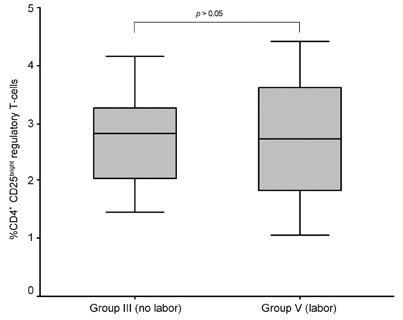
The aim of this study was to evaluate the changes of the regulatory T cell subset in peripheral blood caused by gestational age and premature rupture of membranes (PROM) with or without labor to verify the role of regulatory T cells in pregnancy. PATIENTS AND METHODS: We investigated regulatory T cell distribution in the peripheral blood of pregnancies during the first trimester (group I, n=2), the second trimester (group II, n=12), and the third trimester without PROM and labor (group III, n=15). In addition, we evaluated pregnancies in the third trimester complicated by PROM (group IV, n=4) and labor with no complication by PROM (Group V, n=5). Comparisons were made with non-pregnant controls (group VI, n=4) using flow cytometry. RESULTS: During uncomplicated pregnancy, the CD4(+)CD25(bright) regulatory T cell population decreased with advancing gestational age (group I=3.35+/-0.47, group II=2.91+/-1.44, group III=2.81+/-1.36, group VI=2.52+/-0.71, p=NS). When we compared group IV with group III and V to evaluate the changes of the regulatory T cells with PROM, the CD4(+)CD25(bright) regulatory T cell population was significantly decreased in group IV compared to group III (p=0.001) and group V (p=0.026). CONCLUSION: The present results revealed that the regulatory T cell population increased in early pregnancy but decreased in pregnancies complicated by PROM, indicating that regulatory T cells might be related to the maintenance of pregnancy.
MeSH Terms
Figure
Reference
-
1. Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004. 5:266–271.
Article2. Zenclussen AC, Gerlof K, Zenclussen ML, Sollwedel A, Bertoja AZ, Ritter T, et al. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol. 2005. 166:811–822.
Article3. Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004. 10:347–353.
Article4. Heikkinen J, Möttönen M, Alanen A, Lassila O. Phenotypic characterization of regulatory T cells in the human decidua. Clin Exp Immunol. 2004. 136:373–378.
Article5. Tilburgs T, Roelen DL, van der Mast BJ, van Schip JJ, Kleijburg C, de Groot-Swings GM, et al. Differential distribution of CD4(+)CD25(bright) and CD8(+)CD28(-) T-cells in decidua and maternal blood during human pregnancy. Placenta. 2006. 27:Suppl A. S47–S53.
Article6. Romero R, Chaiworapongsa T, Espinoza J, Gomez R, Yoon BH, Edwin S, et al. Fetal plasma MMP-9 concentrations are elevated in preterm premature rupture of the membranes. Am J Obstet Gynecol. 2002. 187:1125–1130.
Article7. Simhan HN, Caritis SN, Krohn MA, Hillier SL. The vaginal inflammatory milieu and the risk of early premature preterm rupture of membranes. Am J Obstet Gynecol. 2005. 192:213–218.
Article8. Gervasi MT, Chaiworapongsa T, Naccasha N, Pacora P, Berman S, Maymon E, et al. Maternal intravascular inflammation in preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2002. 11:171–175.9. Parry S, Strauss JF 3rd. Premature rupture of the fetal membranes. N Engl J Med. 1998. 338:663–670.
Article10. Gomez R, Ghezzi F, Romero R, Muñoz H, Tolosa JE, Rojas I. Premature labor and intra-amniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clin Perinatol. 1995. 22:281–342.
Article11. Graca L, Thompson S, Lin CY, Adams E, Cobbold SP, Waldmann H. Both CD4(+)CD25(+) and CD4(+)CD25(-) regulatory cells mediate dominant transplantation tolerance. J Immunol. 2002. 168:5558–5565.
Article12. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995. 155:1151–1164.13. Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001. 167:1245–1253.
Article14. Sacks G, Sargent I, Redman C. An innate view of human pregnancy. Immunol Today. 1999. 20:114–118.
Article15. Koumandakis E, Koumandaki I, Kaklamani E, Sparos L, Aravantinos D, Trichopoulos D. Enhanced phagocytosis of mononuclear phagocytes in pregnancy. Br J Obstet Gynaecol. 1986. 93:1150–1154.
Article16. Shibuya T, Izuchi K, Kuroiwa A, Okabe N, Shirakawa K. Study on nonspecific immunity in pregnant women: increased chemiluminescence response of peripheral blood phagocytes. Am J Reprod Immunol Microbiol. 1987. 15:19–23.
Article17. Sargent IL. Maternal and fetal immune responses during pregnancy. Exp Clin Immunogenet. 1993. 10:85–102.18. Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004. 112:38–43.
Article19. Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition--a review. Placenta. 2003. 24:Suppl A. S33–S46.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Peripheral Generation of CD4+ CD25+ Foxp3+ Regulatory T Cells
- Dynamic Frequency of Blood CD4+CD25+ Regulatory T Cells in Rats with Collagen-induced Arthritis
- A Study on the Number of Circulating CD4+CD25+Foxp3+ Regulatory T Cells and CD4+CD25-Foxp3+ T Cells in Psoriasis
- IL-4 Induces CD4+CD25+ Regulatory T Cells from CD4+CD25- T Cells in Peripheral Blood
- Suppression of Pathogenic Autoreactive CD4+ T Cells by CD137-mediated Expansion of CD4+CD25+ Regulatory T Cells in Graves' Disease