Ann Hepatobiliary Pancreat Surg.
2025 May;29(2):113-120. 10.14701/ahbps.24-223.
Neoadjuvant treatment for incidental gallbladder cancer: A systematic review
- Affiliations
-
- 1Department of Surgical Gastroenterology, All India Institute of Medical Sciences, Jodhpur, India
- 2Department of Medical Oncology, Amrita Institute of Medical Sciences, Faridabad, India
- 3Department of Medical Oncology, Vedanta Medical Research Foundation, Balco Medical Center, Raipur, India
- 4Department of Medical Oncology, Memorial Sloan Kettering Cancer Center, New York, NY, United States
- 5Department of Medical Oncology, Weill Cornell College at Cornell University, New York, NY, United States
- 6Department of Medical Oncology, Trinity College Dublin, Dublin, Ireland
- 7Department of Surgical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 8Department of Medical Oncology, Mahatma Gandhi Medical College and Hospital, Jaipur, India
- 9Department of Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 10Department of Visceral Surgery, Krankenhaus Nordwest, Frankfurt, Germany
- 11Department of Visceral Surgery, University Cancer Center Frankfurt, Frankfurt, Germany
- 12Department of Surgical Gastroenterology, Mahatma Gandhi Medical College and Hospital, Jaipur, India
- KMID: 2568259
- DOI: http://doi.org/10.14701/ahbps.24-223
Abstract
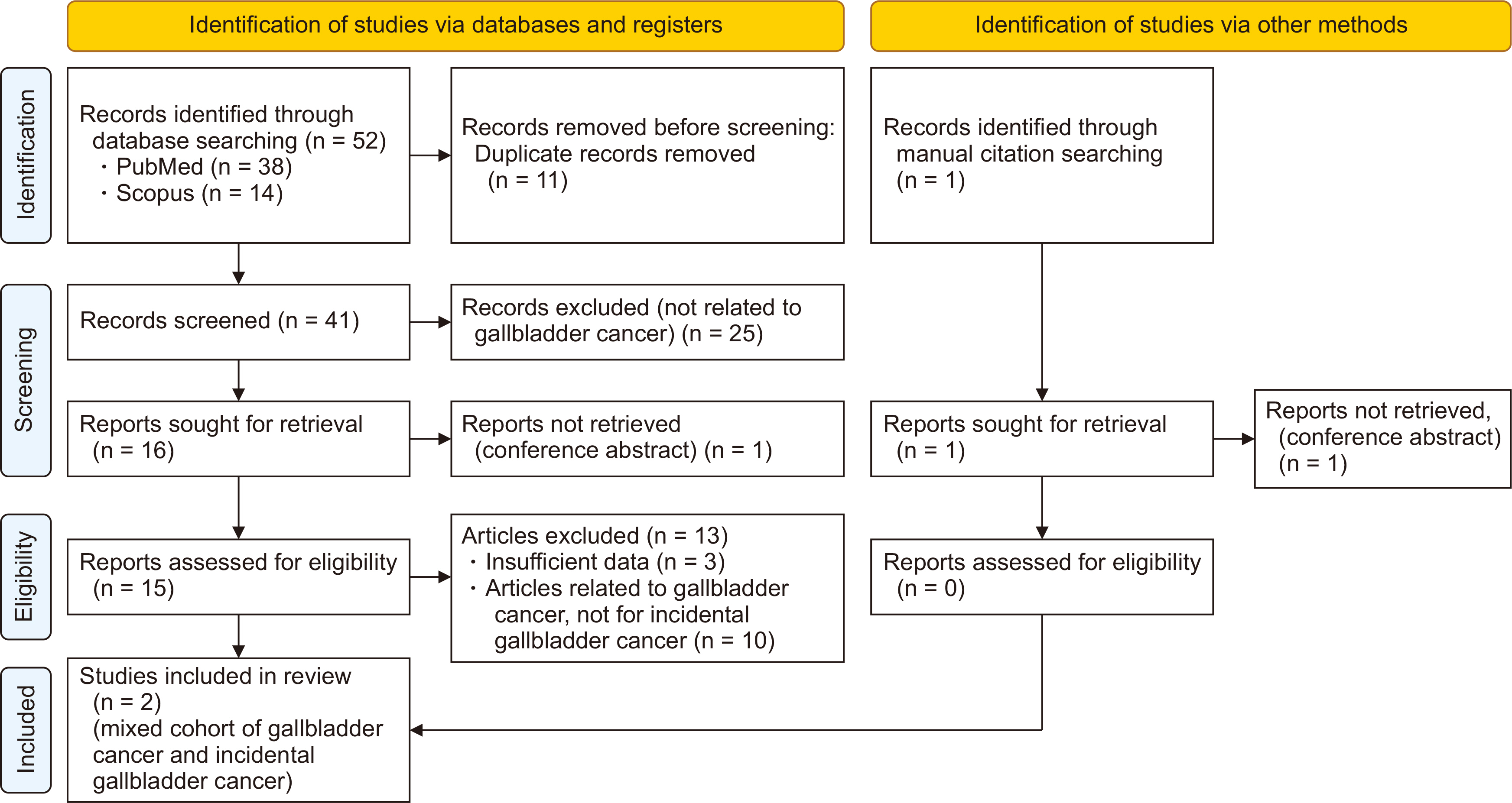
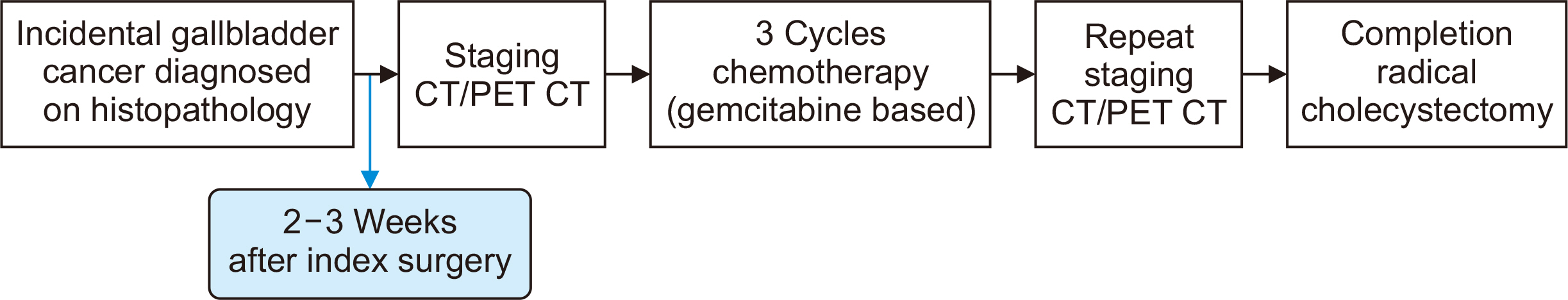
- Incidental gallbladder cancer (iGBC) diagnosed post-histopathological examination of gallbladders removed assuming benign gallstone disease constitutes a significant proportion of GBC patients. Most iGBC patients present with early-stage disease. The standard care for localized (non-metastatic) iGBC includes a reoperation for complete extended (radical) cholecystectomy involving liver resection and lymphadenectomy, followed by postoperative adjuvant systemic therapy. However, a major drawback of this approach is the high recurrence rate within six months post-radical surgery, which undermines the benefits of the extensive procedure; notably, most recurrences are distant, highlighting the efficacy of systemic therapy. Similar to other gastrointestinal cancers, there appears to be a potential for neoadjuvant systemic therapy (chemotherapy) before reoperative surgery in iGBC cases. The premise that neoadjuvant systemic therapy aids in selecting diseases with more favorable biological characteristics and addresses micro-metastatic disease appears applicable to iGBC as well. This systematic review examines the current evidence supporting or refuting neoadjuvant therapy and discusses criteria for selecting patients who would derive significant benefit, along with proposing an optimal chemotherapy regimen for iGBC patients. Improved outcomes have been reported in patients undergoing reoperation after 4 to 14 weeks following the initial cholecystectomy compared to immediate reoperation. Limited, yet promising, evidence supports the use of 3 to 4 cycles of gemcitabine-based neoadjuvant chemotherapy prior to reoperative surgery in select high-risk iGBC cases.
Figure
Reference
-
References
1. Zaidi MY, Abou-Alfa GK, Ethun CG, Shrikhande SV, Goel M, Nervi B, et al. 2019; Evaluation and management of incidental gallbladder cancer. Chin Clin Oncol. 8:37. DOI: 10.21037/cco.2019.07.01. PMID: 31431030. PMCID: PMC8289444.
Article2. Sun J, Xie TG, Ma ZY, Wu X, Li BL. 2023; Current status and progress in laparoscopic surgery for gallbladder carcinoma. World J Gastroenterol. 29:2369–2379. DOI: 10.3748/wjg.v29.i16.2369. PMID: 37179580. PMCID: PMC10167897.
Article3. Benson AB, D'Angelica MI, Abrams T, Abbott DE, Ahmed A, Anaya DA, et al. 2023; NCCN GuidelinesⓇ Insights: biliary tract cancers, version 2.2023. J Natl Compr Canc Netw. 21:694–704. DOI: 10.6004/jnccn.2023.0035. PMID: 37433432.4. Shao J, Lu HC, Wu LQ, Lei J, Yuan RF, Shao JH. 2022; Simple cholecystectomy is an adequate treatment for grade I T1bN0M0 gallbladder carcinoma: evidence from 528 patients. World J Gastroenterol. 28:4431–4441. DOI: 10.3748/wjg.v28.i31.4431. PMID: 36159006. PMCID: PMC9453773.
Article5. Matsuyama R, Yabusita Y, Homma Y, Kumamoto T, Endo I. Essential updates 2019/2020: surgical treatment of gallbladder cancer. Ann Gastroenterol Surg. 2021; 5:152–161. DOI: 10.1002/ags3.12434. PMID: 33860135. PMCID: PMC8034687.
Article6. Patkar S, Patel S, Gupta A, Ramaswamy A, Ostwal V, Goel M. 2021; Revision surgery for incidental gallbladder cancer-challenging the dogma: ideal timing and real-world applicability. Ann Surg Oncol. 28:6758–6766. DOI: 10.1245/s10434-021-09687-4. PMID: 33625635.
Article7. Foster JM, Hoshi H, Gibbs JF, Iyer R, Javle M, Chu Q, et al. 2007; Gallbladder cancer: Defining the indications for primary radical resection and radical re-resection. Ann Surg Oncol. 14:833–840. DOI: 10.1245/s10434-006-9097-6. PMID: 17103074.
Article8. Goetze TO, Paolucci V. 2010; Adequate extent in radical re-resection of incidental gallbladder carcinoma: analysis of the German Registry. Surg Endosc. 24:2156–2164. DOI: 10.1007/s00464-010-0914-4. PMID: 20177938.
Article9. Ouchi K, Mikuni J, Kakugawa Y. 2002; Laparoscopic cholecystectomy for gallbladder carcinoma: results of a Japanese survey of 498 patients. J Hepatobiliary Pancreat Surg. 9:256–260. DOI: 10.1007/s005340200028. PMID: 12140616.
Article10. Shirai Y, Yoshida K, Tsukada K, Muto T. 1992; Inapparent carcinoma of the gallbladder. An appraisal of a radical second operation after simple cholecystectomy. Ann Surg. 215:326–331. DOI: 10.1097/00000658-199204000-00004. PMID: 1558412. PMCID: PMC1242447.11. Cherkassky L, D'Angelica M. 2019; Gallbladder cancer: managing the incidental diagnosis. Surg Oncol Clin N Am. 28:619–630. DOI: 10.1016/j.soc.2019.06.005. PMID: 31472909.12. Lundgren L, Muszynska C, Ros A, Persson G, Gimm O, Andersson B, et al. 2019; Management of incidental gallbladder cancer in a national cohort. Br J Surg. 106:1216–1227. DOI: 10.1002/bjs.11205. PMID: 31259388.
Article13. Feo CF, Ginesu GC, Fancellu A, Perra T, Ninniri C, Deiana G, et al. 2022; Current management of incidental gallbladder cancer: a review. Int J Surg. 98:106234. DOI: 10.1016/j.ijsu.2022.106234. PMID: 35074510.
Article14. Shirai Y, Yoshida K, Tsukada K, Muto T, Watanabe H. 1992; Radical surgery for gallbladder carcinoma. Long-term results. Ann Surg. 216:565–568. DOI: 10.1097/00000658-199211000-00007. PMID: 1359844. PMCID: PMC1242674.
Article15. Fong Y, Jarnagin W, Blumgart LH. 2000; Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg. 232:557–569. DOI: 10.1097/00000658-200010000-00011. PMID: 10998654. PMCID: PMC1421188.
Article16. Horkoff MJ, Ahmed Z, Xu Y, Sutherland FR, Dixon E, Ball CG, et al. 2021; Adverse outcomes after bile spillage in incidental gallbladder cancers: a population-based study. Ann Surg. 273:139–144. DOI: 10.1097/SLA.0000000000003325. PMID: 30998534.17. Butte JM, Waugh E, Meneses M, Parada H, De La Fuente HA. 2010; Incidental gallbladder cancer: analysis of surgical findings and survival. J Surg Oncol. 102:620–625. DOI: 10.1002/jso.21681. PMID: 20721958.
Article18. Leung U, Pandit-Taskar N, Corvera CU, D'Angelica MI, Allen PJ, Kingham TP, et al. 2014; Impact of pre-operative positron emission tomography in gallbladder cancer. HPB (Oxford). 16:1023–1030. DOI: 10.1111/hpb.12282. PMID: 24894161. PMCID: PMC4487754.
Article19. D'Angelica M, Dalal KM, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. 2009; Analysis of the extent of resection for adenocarcinoma of the gallbladder. Ann Surg Oncol. 16:806–816. DOI: 10.1245/s10434-008-0189-3. PMID: 18985272.20. Ito H, Ito K, D'Angelica M, Gonen M, Klimstra D, Allen P, et al. 2011; Accurate staging for gallbladder cancer: implications for surgical therapy and pathological assessment. Ann Surg. 254:320–325. DOI: 10.1097/SLA.0b013e31822238d8. PMID: 21617582.21. Tran TB, Nissen NN. 2015; Surgery for gallbladder cancer in the US: a need for greater lymph node clearance. J Gastrointest Oncol. 6:452–458.22. Shindoh J, de Aretxabala X, Aloia TA, Roa JC, Roa I, Zimmitti G, et al. 2015; Tumor location is a strong predictor of tumor progression and survival in T2 gallbladder cancer: an international multicenter study. Ann Surg. 261:733–739. DOI: 10.1097/SLA.0000000000000728. PMID: 24854451. PMCID: PMC4800978.
Article23. Chun YS, Pawlik TM, Vauthey JN. 2018; 8th edition of the AJCC Cancer Staging Manual: pancreas and hepatobiliary cancers. Ann Surg Oncol. 25:845–847. DOI: 10.1245/s10434-017-6025-x. PMID: 28752469.
Article24. Horgan AM, Amir E, Walter T, Knox JJ. 2012; Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol. 30:1934–1940. DOI: 10.1200/JCO.2011.40.5381. PMID: 22529261.
Article25. Butte JM, Kingham TP, Gönen M, D'Angelica MI, Allen PJ, Fong Y, et al. 2014; Residual disease predicts outcomes after definitive resection for incidental gallbladder cancer. J Am Coll Surg. 219:416–429. DOI: 10.1016/j.jamcollsurg.2014.01.069. PMID: 25087941. PMCID: PMC4143454.
Article26. Ma N, Cheng H, Qin B, Zhong R, Wang B. 2015; Adjuvant therapy in the treatment of gallbladder cancer: a meta-analysis. BMC Cancer. 15:615. DOI: 10.1186/s12885-015-1617-y. PMID: 26337466. PMCID: PMC4559875.
Article27. Ethun CG, Le N, Lopez-Aguiar AG, Pawlik TM, Poultsides G, Tran T, et al. 2017; Pathologic and Prognostic Implications of Incidental versus Nonincidental gallbladder cancer: a 10-institution study from the United States extrahepatic biliary malignancy consortium. Am Surg. 83:679–686. DOI: 10.1177/000313481708300721. PMID: 28738935. PMCID: PMC5915617.
Article28. Goetze TO, Paolucci V. 2008; Benefits of reoperation of T2 and more advanced incidental gallbladder carcinoma: analysis of the German registry. Ann Surg. 247:104–108. DOI: 10.1097/SLA.0b013e318154bf5d. PMID: 18156929.
Article29. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. ABC-02 Trial Investigators. 2010; Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 362:1273–1281. DOI: 10.1056/NEJMoa0908721. PMID: 20375404.
Article30. Shroff RT, King G, Colby S, Scott AJ, Borad MJ, Goff L, et al. SWOG S1815: a phase iii randomized trial of gemcitabine, cisplatin, and nab-paclitaxel versus gemcitabine and cisplatin in newly diagnosed, advanced biliary tract cancers. J Clin Oncol. 2025; 43:536–544. DOI: 10.1200/JCO-24-01383. PMID: 39671534. PMCID: PMC11798714.
Article31. Shroff RT, Javle MM, Xiao L, Kaseb AO, Varadhachary GR, Wolff RA, et al. 2019; Gemcitabine, cisplatin, and nab-paclitaxel for the treatment of advanced biliary tract cancers: a phase 2 clinical trial. JAMA Oncol. 5:824–830. DOI: 10.1001/jamaoncol.2019.0270. PMID: 30998813. PMCID: PMC6567834.
Article32. Gedela S, Munot P, Vaidyanathan A, Joarder R, Chaugule D, Parulekar M, et al. 2024; Gemcitabine, cisplatin, and nab-paclitaxel as a first-line therapy for advanced biliary tract cancers. J Gastrointest Cancer. 55:263–269. DOI: 10.1007/s12029-023-00946-z. PMID: 37368175.
Article33. Phelip JM, Desrame J, Edeline J, Barbier E, Terrebonne E, Michel P, et al. PRODIGE 38 AMEBICA Investigators/Collaborators. 2022; Modified FOLFIRINOX versus CISGEM chemotherapy for patients with advanced biliary tract cancer (PRODIGE 38 AMEBICA): a randomized phase II study. J Clin Oncol. 40:262–271. DOI: 10.1200/JCO.21.00679. PMID: 34662180.
Article34. Oh DY, Ruth He A, Qin S, Chen LT, Okusaka T, Vogel A, et al. 2022; Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 1:EVIDoa2200015. DOI: 10.1056/EVIDoa2200015. PMID: 38319896.
Article35. Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, et al. KEYNOTE-966 Investigators. 2023; Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 401:1853–1865. DOI: 10.1016/S0140-6736(23)00727-4. PMID: 37075781.36. Chaudhari VA, Ostwal V, Patkar S, Sahu A, Toshniwal A, Ramaswamy A, et al. 2018; Outcome of neoadjuvant chemotherapy in "locally advanced/borderline resectable" gallbladder cancer: the need to define indications. HPB (Oxford). 20:841–847. DOI: 10.1016/j.hpb.2018.03.008. PMID: 29706425.
Article37. Creasy JM, Goldman DA, Dudeja V, Lowery MA, Cercek A, Balachandran VP, et al. 2017; Systemic chemotherapy combined with resection for locally advanced gallbladder carcinoma: surgical and survival outcomes. J Am Coll Surg. 224:906–916. DOI: 10.1016/j.jamcollsurg.2016.12.058. PMID: 28216422. PMCID: PMC5409857.
Article38. Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, et al. BILCAP study group. 2019; Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 20:663–673. DOI: 10.1016/S1470-2045(18)30915-X. PMID: 30922733.
Article39. Naveed S, Qari H, Thau CM, Burasakarn P, Mir AW. 2021; Neoadjuvant chemotherapy for advanced gallbladder cancer: do we have enough evidence? A systematic review. Euroasian J Hepatogastroenterol. 11:87–94. DOI: 10.5005/jp-journals-10018-1348. PMID: 34786362. PMCID: PMC8566156.
Article40. Goetze TO, Lorenzen S, Bankstahl US, Lang SA, Wittel UA, Heeg S, et al. 2021; Perioperative chemotherapy with gemcitabine plus cisplatin followed by radical liver resection versus immediate radical liver resection alone in gallbladder carcinoma or in front of radical resection in BTC: the phase III GAIN trial. J Clin Oncol. 39(3 suppl):TPS353. DOI: 10.1200/JCO.2021.39.3_suppl.TPS353.
Article41. Ben-Josef E, Guthrie KA, El-Khoueiry AB, Corless CL, Zalupski MM, Lowy AM, et al. SWOG S0809: a phase II intergroup trial of adjuvant capecitabine and gemcitabine followed by radiotherapy and concurrent capecitabine in extrahepatic cholangiocarcinoma and gallbladder carcinoma. J Clin Oncol. 2015; 33:2617–2622. DOI: 10.1200/JCO.2014.60.2219. PMID: 25964250. PMCID: PMC4534524.
Article42. Hakeem AR, Papoulas M, Menon KV. 2019; The role of neoadjuvant chemotherapy or chemoradiotherapy for advanced gallbladder cancer - a systematic review. Eur J Surg Oncol. 45:83–91. DOI: 10.1016/j.ejso.2018.08.020. PMID: 30287098.
Article43. de Aretxabala X, Losada H, Mora J, Roa I, Burgos L, Yáñez E, et al. 2004; [Neoadjuvant chemoradiotherapy in gallbladder cancer]. Rev Med Chil. 132:51–57. Spanish. DOI: 10.4067/S0034-98872004000100008. PMID: 15379053.44. Morganti AG, Trodella L, Valentini V, Montemaggi P, Costamagna G, Smaniotto D, et al. 2000; Combined modality treatment in unresectable extrahepatic biliary carcinoma. Int J Radiat Oncol Biol Phys. 46:913–919. DOI: 10.1016/S0360-3016(99)00487-3. PMID: 10705013.
Article45. Engineer R, Patkar S, Lewis SC, Sharma AD, Shetty N, Ostwal V, et al. 2019; A phase III randomised clinical trial of perioperative therapy (neoadjuvant chemotherapy versus chemoradiotherapy) in locally advanced gallbladder cancers (POLCAGB): study protocol. BMJ Open. 9:e028147. DOI: 10.1136/bmjopen-2018-028147. PMID: 31253621. PMCID: PMC6609079.
Article46. Jarnagin WR, Ruo L, Little SA, Klimstra D, D'Angelica M, DeMatteo RP, et al. 2003; Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 98:1689–1700. DOI: 10.1002/cncr.11699. PMID: 14534886.
Article47. Ostwal V, Patkar S, Engineer R, Parulekar M, Mandavkar S, Bhargava P, et al. 2024; Adjuvant gemcitabine plus cisplatin and chemoradiation in patients with gallbladder cancer: a randomized clinical trial. JAMA Oncol. 10:1116–1120. DOI: 10.1001/jamaoncol.2024.1944. PMID: 38958997.
Article48. Rahul R, Haldenia K, Singh A, Kapoor V, Singh RK, Saxena R. 2022; does timing of completion radical cholecystectomy determine the survival outcome in incidental carcinoma gallbladder: a single-center retrospective analysis. Cureus. 14:e26653. DOI: 10.7759/cureus.26653.
Article49. Javle M, Borad MJ, Azad NS, Kurzrock R, Abou-Alfa GK, George B, et al. 2021; Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 22:1290–1300. DOI: 10.1016/S1470-2045(21)00336-3. PMID: 34339623.
Article50. Das CK, Patel A, Raj A, Gupta VG, Mehta P, Bhethanabhotla S, et al. 2020; Anti-Her2neu directed therapy in advanced gall bladder cancer: a prospective, multicenter experience from India. J Clin Oncol. 38(15 suppl):e16682. DOI: 10.1200/JCO.2020.38.15_suppl.e16682.
Article51. Javle M, Churi C, Kang HC, Shroff R, Janku F, Surapaneni R, et al. 2015; HER2/neu-directed therapy for biliary tract cancer. J Hematol Oncol. 8:58. DOI: 10.1186/s13045-015-0155-z. PMID: 26022204. PMCID: PMC4469402.
Article52. Lamarca A, Barriuso J, McNamara MG, Valle JW. 2018; Biliary tract cancer: state of the art and potential role of DNA damage repair. Cancer Treat Rev. 70:168–177. DOI: 10.1016/j.ctrv.2018.09.002. PMID: 30218788.
Article53. Ueno M, Chung HC, Nagrial A, Marabelle A, Kelley RK, Xu L, et al. 2018; Pembrolizumab for advanced biliary adenocarcinoma: Results from the multicohort, phase II KEYNOTE-158 study. Ann Oncol. 29(Suppl 8):viii210. DOI: 10.1093/annonc/mdy282.009.
Article54. Ikeda M, Ueno M, Morizane C, Kobayashi S, Ohno I, Kondo S, et al. 2019; A multicenter, open-label, phase I study of nivolumab alone or in combination with gemcitabine plus cisplatin in patients with unresectable or recurrent biliary tract cancer. J Clin Oncol. 37(4 suppl):306. DOI: 10.1200/JCO.2019.37.4_suppl.306.
Article55. Oh DY, Lee KH, Lee DW, Kim TY, Bang JH, Nam AR, et al. 2020; Phase II study assessing tolerability, efficacy, and biomarkers for durvalumab (D) ± tremelimumab (T) and gemcitabine/cisplatin (GemCis) in chemo-naïve advanced biliary tract cancer (aBTC). J Clin Oncol. 38(15 suppl):4520. DOI: 10.1200/JCO.2020.38.15_suppl.4520.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reviewing the potential role of radiation therapy in gallbladder cancer: an update
- Interpretation of Rectal MRI after Neoadjuvant Treatment in Patients with Rectal Cancer
- A Case of Synchronous Double Primary Cancer Associated with the Biliary Tract
- Cholelithiasis as a Risk Factor for Gallbladder Cancer
- When Do We Need Reoperation in Incidental Gallbladder Cancer after Laparoscopic Cholecystectomy?