Cancer Res Treat.
2025 Apr;57(2):558-569. 10.4143/crt.2024.127.
Survival of Children with Acute Lymphoblastic Leukemia with Risk Group–Based Protocol Changes: A Single-Center Experience with 460 Patients over a 20-Year Period
- Affiliations
-
- 1Department of Pediatrics, Cha Bundang Medical Center, Cha University, Seongnam, Korea
- 2Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Department of Pediatrics, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
- 4Department of Pediatrics, Ajou University Hospital, Ajou University School of Medicine, Suwon, Korea
- 5Korea Hemophilia Foundation, Seoul, Korea
- KMID: 2566872
- DOI: http://doi.org/10.4143/crt.2024.127
Abstract
- Purpose
Recent treatments for pediatric acute lymphoblastic leukemia (ALL) are founded on risk stratification. We examined the survival rates and prognostic factors of patients over a 20-year period at a single institution.
Materials and Methods
This study analyzed patients diagnosed with ALL and treated at the Pediatric Department of Samsung Medical Center (SMC). Patients were categorized into standard-risk (SR), high-risk (HR), and very high-risk (VHR) groups. The SMC protocol for the HR group underwent two changes during the study period: a modified Children’s Cancer Group (CCG)-1882 protocol was used from 2000 to 2005, the Korean multicenter HR ALL-0601 protocol from 2006 to 2014, and the Korean multicenter HR ALL-1501 protocol from 2015 to 2019.
Results
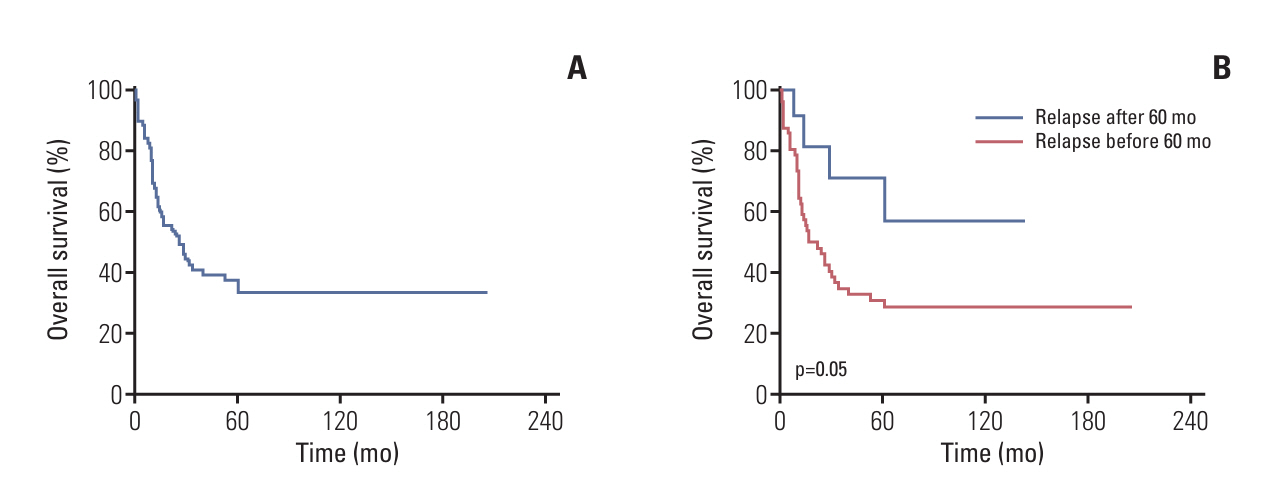
Of the 460 patients, complete remission was achieved in 436 patients (94.8%). The 10-year overall survival rate (OS) was 83.8±1.9% for all patients. OS according to the SMC risk group was as follows: 95.9%±1.4% in the SR group, 83.8%±3.6% in the HR group, and 66.2%±6.9% in the VHR group. The 5-year OS within the HR group varied according to the treatment protocol: 73.9%±7.5%, in the modified CCG-1882 protocol, 83.0%±3.9%, in the 0601 protocol, and 96.2%±2.6%, in the 1501 protocol. For those aged 15 years and older, the OS was only 56.5%±13.1%. Relapse occurred in 71 patients (15.4%), and the OS after relapse was 37.7%±6.0%.
Conclusion
The treatment outcomes of patients with ALL improved markedly. However, there is a need to further characterize adolescents and young adult patients, as well as those who have experienced relapses.
Figure
Reference
-
References
1. Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015; 373:1541–52.
Article2. Inaba H, Mullighan CG. Pediatric acute lymphoblastic leukemia. Haematologica. 2020; 105:2524–39.
Article3. Stanulla M, Schrappe M. Treatment of childhood acute lymphoblastic leukemia. Semin Hematol. 2009; 46:52–63.
Article4. Moricke A, Zimmermann M, Valsecchi MG, Stanulla M, Biondi A, Mann G, et al. Dexamethasone vs prednisone in induction treatment of pediatric ALL: results of the randomized trial AIEOP-BFM ALL 2000. Blood. 2016; 127:2101–12.
Article5. Larsen EC, Devidas M, Chen S, Salzer WL, Raetz EA, Loh ML, et al. Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk B-acute lymphoblastic leukemia: a report from Children’s Oncology Group Study AALL0232. J Clin Oncol. 2016; 34:2380–8.
Article6. Winter SS, Dunsmore KP, Devidas M, Wood BL, Esiashvili N, Chen Z, et al. Improved survival for children and young adults with T-lineage acute lymphoblastic leukemia: results from the Children’s Oncology Group AALL0434 methotrexate randomization. J Clin Oncol. 2018; 36:2926–34.
Article7. Jeha S, Pei D, Choi J, Cheng C, Sandlund JT, Coustan-Smith E, et al. Improved CNS control of childhood acute lymphoblastic leukemia without cranial irradiation: St Jude Total Therapy Study 16. J Clin Oncol. 2019; 37:3377–91.
Article8. Schultz KR, Pullen DJ, Sather HN, Shuster JJ, Devidas M, Borowitz MJ, et al. Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children’s Cancer Group (CCG). Blood. 2007; 109:926–35.
Article9. Lange BJ, Bostrom BC, Cherlow JM, Sensel MG, La MK, Rackoff W, et al. Double-delayed intensification improves event-free survival for children with intermediate-risk acute lymphoblastic leukemia: a report from the Children’s Cancer Group. Blood. 2002; 99:825–33.
Article10. Nachman JB, Sather HN, Sensel MG, Trigg ME, Cherlow JM, Lukens JN, et al. Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med. 1998; 338:1663–71.
Article11. Moricke A, Reiter A, Zimmermann M, Gadner H, Stanulla M, Dordelmann M, et al. Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood. 2008; 111:4477–89.12. Maloney KW, Devidas M, Wang C, Mattano LA, Friedmann AM, Buckley P, et al. Outcome in children with standard-risk B-cell acute lymphoblastic leukemia: results of Children’s Oncology Group Trial AALL0331. J Clin Oncol. 2020; 38:602–12.
Article13. Steinherz PG, Seibel NL, Sather H, Ji L, Xu X, Devidas M, et al. Treatment of higher risk acute lymphoblastic leukemia in young people (CCG-1961), long-term follow-up: a report from the Children’s Oncology Group. Leukemia. 2019; 33:2144–54.
Article14. Toft N, Birgens H, Abrahamsson J, Griskevicius L, Hallbook H, Heyman M, et al. Results of NOPHO ALL2008 treatment for patients aged 1-45 years with acute lymphoblastic leukemia. Leukemia. 2018; 32:606–15.
Article15. Pedrosa F, Coustan-Smith E, Zhou Y, Cheng C, Pedrosa A, Lins MM, et al. Reduced-dose intensity therapy for pediatric lymphoblastic leukemia: long-term results of the Recife RELLA05 pilot study. Blood. 2020; 135:1458–66.
Article16. Podpeskar A, Crazzolara R, Kropshofer G, Obexer P, Rabensteiner E, Michel M, et al. Supportive methods for childhood acute lymphoblastic leukemia then and now: a compilation for clinical practice. Front Pediatr. 2022; 10:980234.
Article17. Wood B, Winter S, Dunsmore K, Raetz E, Borowitz MJ, Devidas M, et al. Patients with early T-cell precursor (ETP) acute lymphoblastic leukemia (ALL) have high levels of minimal residual disease (MRD) at the end of induction: a Children’s Oncology Group (COG) study. Blood. 2009; 114:9.
Article18. Inaba H, Pui CH. Advances in the diagnosis and treatment of pediatric acute lymphoblastic leukemia. J Clin Med. 2021; 10:1926.
Article19. Gaynon PS, Bleyer WA, Steinherz PG, Finklestein JZ, Littman P, Miller DR, et al. Day 7 marrow response and outcome for children with acute lymphoblastic leukemia and unfavorable presenting features. Med Pediatr Oncol. 1990; 18:273–9.
Article20. Yoon JH, Kim EK, Park JA, Kang HJ, Shin HY, Ahn HS. Effect of treatment modification by initial response in high-risk childhood acute lymphoblastic leukemia. Korean J Hematol. 2008; 43:238–46.
Article21. Schrappe M, Reiter A, Zimmermann M, Harbott J, Ludwig WD, Henze G, et al. Long-term results of four consecutive trials in childhood ALL performed by the ALL-BFM study group from 1981 to 1995. Berlin-Frankfurt-Munster. Leukemia. 2000; 14:2205–22.
Article22. Seibel NL, Steinherz PG, Sather HN, Nachman JB, Delaat C, Ettinger LJ, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Blood. 2008; 111:2548–55.
Article23. Larsen E, Salzer WL, Devidas M, Nachman JB, Raetz EA, Loh ML, et al. Comparison of high-dose methotrexate (HD-MTX) with Capizzi methotrexate plus asparaginase (C-MTX/ASNase) in children and young adults with high-risk acute lymphoblastic leukemia (HR-ALL): a report from the Children’s Oncology Group Study AALL0232. J Clin Oncol. 2011; 29(18 Suppl):3.
Article24. Lee JW. Optimal therapy for adolescents and young adults with acute lymphoblastic leukemia-current perspectives. Blood Res. 2020; 55:S27–31.
Article25. Boissel N. New developments in ALL in AYA. Hematology Am Soc Hematol Educ Program. 2022; 2022:190–6.
Article26. Hough R, Rowntree C, Goulden N, Mitchell C, Moorman A, Wade R, et al. Efficacy and toxicity of a paediatric protocol in teenagers and young adults with Philadelphia chromosome negative acute lymphoblastic leukaemia: results from UKALL 2003. Br J Haematol. 2016; 172:439–51.
Article27. Chessells JM. Relapsed lymphoblastic leukaemia in children: a continuing challenge. Br J Haematol. 1998; 102:423–38.
Article28. Kato M, Manabe A, Saito AM, Koh K, Inukai T, Ogawa C, et al. Outcome of pediatric acute lymphoblastic leukemia with very late relapse: a retrospective analysis by the Tokyo Children’s Cancer Study Group (TCCSG). Int J Hematol. 2015; 101:52–7.
Article29. Rizzari C, Valsecchi MG, Arico M, Miniero R, Messina C, De Rossi G, et al. Outcome of very late relapse in children with acute lymphoblastic leukemia. Haematologica. 2004; 89:427–34.30. Aldoss I, Pillai R, Yang D, Yang L, Arslan S, Mokhtari S, et al. Late and very late relapsed acute lymphoblastic leukemia: clinical and molecular features, and treatment outcomes. Blood Cancer J. 2021; 11:125.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epidemiology of Acute Leukemia among Children with Down Syndrome in Korea
- Survival Benefit of Hematopoietic Stem Cell Transplantation in Infant Acute Lymphoblastic Leukemia, According to Classification of High Risk Group
- Advances in the Treatment of Childhood Acute Lymphoblastic Leukemia
- Precursor B-Cell Acute Lymphoblastic Leukemia in Two Patients with a History of Cytotoxic Therapy
- Myeloablative Hematopoietic Stem Cell Transplantation with a Non-total Body Irradiation Regimen for Treating Pediatric Acute Lymphoblastic Leukemia