Cancer Res Treat.
2025 Apr;57(2):401-411. 10.4143/crt.2024.670.
Histological Assessment and Interobserver Agreement in Major Pathologic Response for Non–Small Cell Lung Cancer with Neoadjuvant Therapy
- Affiliations
-
- 1Department of Translational Medicine, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Pathology and Translational Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 3Department of Pathology, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2566859
- DOI: http://doi.org/10.4143/crt.2024.670
Abstract
- Purpose
Major pathologic response (MPR), defined as ≤ 10% of residual viable tumor (VT), is a prognostic factor in non–small cell lung cancer (NSCLC) after neoadjuvant therapy. This study evaluated interobserver reproducibility in assessing MPR, compared area-weighted and unweighted VT (%) calculation, and determined optimal VT (%) cutoffs across histologic subtypes for survival prediction.
Materials and Methods
This retrospective study included 108 patients with NSCLC who underwent surgical resection after neoadjuvant chemotherapy or chemoradiation at Seoul National University Bundang Hospital between 2009-2018. Three observers with varying expertise independently assessed tumor bed and VT (%) based on digital whole-slide images.
Results
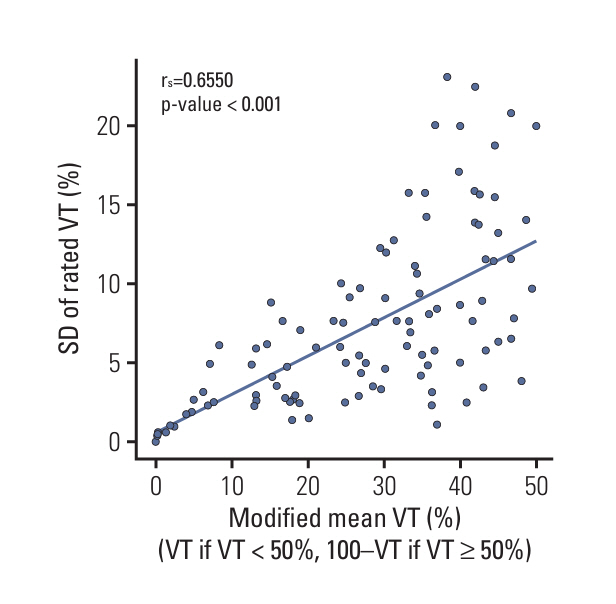
Reproducibility in tumor bed delineation was reduced in squamous cell carcinoma (SqCC) with smaller tumor bed, although overall concordance was high (Dice coefficient, 0.96; intersection-over-union score, 0.92). Excellent agreement was achieved for VT (%) (intraclass correlation coefficient=0.959) and MPR using 10% cutoff (Fleiss’ kappa=0.911). Shifting between area-weighted and unweighted VT (%) showed only one case differing in MPR status out of 81 cases. The optimal cutoff was 10% for both adenocarcinoma (ADC) and SqCC. MPR+ was observed in 18 patients (17%), with SqCC showing higher MPR+ rates (p=0.044), lower VT (%) (p < 0.001), and better event-free survival (p=0.015) than ADC. MPR+ significantly improved overall survival (p=0.023), event-free survival (p=0.001), and lung cancer-specific survival (p=0.012).
Conclusion
While MPR assessment demonstrated robust reproducibility with minimal impact from the tumor bed, attention is warranted when evaluating smaller tumor beds in SqCC. A 10% cutoff reliably predicted survival across histologic subtypes with higher interobserver reproducibility.
Keyword
Figure
Reference
-
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–49.
Article2. Leiter A, Veluswamy RR, Wisnivesky JP. The global burden of lung cancer: current status and future trends. Nat Rev Clin Oncol. 2023; 20:624–39.
Article3. Song WA, Zhou NK, Wang W, Chu XY, Liang CY, Tian XD, et al. Survival benefit of neoadjuvant chemotherapy in non-small cell lung cancer: an updated meta-analysis of 13 randomized control trials. J Thorac Oncol. 2010; 5:510–6.
Article4. Yau C, Osdoit M, van der Noordaa M, Shad S, Wei J, de Croze D, et al. Residual cancer burden after neoadjuvant chemotherapy and long-term survival outcomes in breast cancer: a multicentre pooled analysis of 5161 patients. Lancet Oncol. 2022; 23:149–60.5. Menzies AM, Amaria RN, Rozeman EA, Huang AC, Tetzlaff MT, van de Wiel BA, et al. Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). Nat Med. 2021; 27:301–9.
Article6. Pietrasz D, Marthey L, Wagner M, Blanc JF, Laurent C, Turrini O, et al. Pathologic major response after FOLFIRINOX is prognostic for patients secondary resected for borderline or locally advanced pancreatic adenocarcinoma: an AGEO-FRENCH, prospective, multicentric cohort. Ann Surg Oncol. 2015; 22 Suppl 3:S1196–205.
Article7. Petrelli F, Coinu A, Cabiddu M, Ghilardi M, Vavassori I, Barni S. Correlation of pathologic complete response with survival after neoadjuvant chemotherapy in bladder cancer treated with cystectomy: a meta-analysis. Eur Urol. 2014; 65:350–7.
Article8. Junker K, Thomas M, Schulmann K, Klinke F, Bosse U, Muller KM. Tumour regression in non-small-cell lung cancer following neoadjuvant therapy. Histological assessment. J Cancer Res Clin Oncol. 1997; 123:469–77.
Article9. Junker K, Langner K, Klinke F, Bosse U, Thomas M. Grading of tumor regression in non-small cell lung cancer: morphology and prognosis. Chest. 2001; 120:1584–91.
Article10. Pataer A, Kalhor N, Correa AM, Raso MG, Erasmus JJ, Kim ES, et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol. 2012; 7:825–32.
Article11. Hellmann MD, Chaft JE, William WN Jr, Rusch V, Pisters KM, Kalhor N, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol. 2014; 15:e42–50.
Article12. Catania C, Muthusamy B, Spitaleri G, Del Signore E, Pennell NA. The new era of immune checkpoint inhibition and target therapy in early-stage non-small cell lung cancer: a review of the literature. Clin Lung Cancer. 2022; 23:108–15.
Article13. Travis WD, Dacic S, Wistuba I, Sholl L, Adusumilli P, Bubendorf L, et al. IASLC Multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens after neoadjuvant therapy. J Thorac Oncol. 2020; 15:709–40.
Article14. Saqi A, Leslie KO, Moreira AL, Lantuejoul S, Shu CA, Rizvi NA, et al. Assessing pathologic response in resected lung cancers: current standards, proposal for a novel pathologic response calculator tool, and challenges in practice. JTO Clin Res Rep. 2022; 3:100310.
Article15. Qu Y, Emoto K, Eguchi T, Aly RG, Zheng H, Chaft JE, et al. Pathologic assessment after neoadjuvant chemotherapy for NSCLC: importance and implications of distinguishing adenocarcinoma from squamous cell carcinoma. J Thorac Oncol. 2019; 14:482–93.
Article16. Zens P, Bello C, Scherz A, Koenigsdorf J, Pollinger A, Schmid RA, et al. A prognostic score for non-small cell lung cancer resected after neoadjuvant therapy in comparison with the tumor-node-metastases classification and major pathological response. Mod Pathol. 2021; 34:1333–44.
Article17. Liu X, Sun W, Wu J, Feng Y, Mao L, Chen M, et al. Major pathologic response assessment and clinical significance of metastatic lymph nodes after neoadjuvant therapy for non-small cell lung cancer. Mod Pathol. 2021; 34:1990–8.
Article18. Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. Springer;2017.19. Dacic S, Travis W, Redman M, Saqi A, Cooper WA, Borczuk A, et al. International Association for the Study of Lung Cancer Study of reproducibility in assessment of pathologic response in resected lung cancers after neoadjuvant therapy. J Thorac Oncol. 2023; 18:1290–302.
Article20. Dacic S, Travis WD, Giltnane JM, Kos F, Abel J, Hilz S, et al. Artificial intelligence-powered assessment of pathologic response to neoadjuvant atezolizumab in patients With NSCLC: results from the LCMC3 study. J Thorac Oncol. 2024; 19:719–31.
Article21. Cai JS, Li S, Yan SM, Yang J, Yang MZ, Xie CL, et al. Is major pathologic response sufficient to predict survival in resectable nonsmall-cell lung cancer patients receiving neoadjuvant chemotherapy? Thorac Cancer. 2021; 12:1336–46.
Article22. Liu-Jarin X, Stoopler MB, Raftopoulos H, Ginsburg M, Gorenstein L, Borczuk AC. Histologic assessment of non-small cell lung carcinoma after neoadjuvant therapy. Mod Pathol. 2003; 16:1102–8.
Article23. Liao WY, Chen JH, Wu M, Shih JY, Chen KY, Ho CC, et al. Neoadjuvant chemotherapy with docetaxel-cisplatin in patients with stage III N2 non-small-cell lung cancer. Clin Lung Cancer. 2013; 14:418–24.
Article24. Haque W, Verma V, Butler EB, Teh BS. Pathologic nodal clearance and complete response following neoadjuvant chemoradiation for clinical N2 non-small cell lung cancer: predictors and long-term outcomes. Lung Cancer. 2019; 130:93–100.
Article25. Pottgen C, Stuschke M, Graupner B, Theegarten D, Gauler T, Jendrossek V, et al. Prognostic model for long-term survival of locally advanced non-small-cell lung cancer patients after neoadjuvant radiochemotherapy and resection integrating clinical and histopathologic factors. BMC Cancer. 2015; 15:363.
Article26. Schreiner W, Dudek W, Rieker RJ, Lettmaier S, Fietkau R, Sirbu H. Major pathologic response after induction therapy has a long-term impact on survival and tumor recurrence in stage IIIA/B locally advanced NSCLC. Thorac Cardiovasc Surg. 2020; 68:639–45.
Article27. Thomas M, Rube C, Hoffknecht P, Macha HN, Freitag L, Linder A, et al. Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomised trial in stage III non-small-cell lung cancer. Lancet Oncol. 2008; 9:636–48.
Article28. Munoz-Guglielmetti D, Sanchez-Lorente D, Reyes R, Martinez D, Lucena C, Boada M, et al. Pathological response to neoadjuvant therapy with chemotherapy vs chemoradiotherapy in stage III NSCLC-contribution of IASLC recommendations. World J Clin Oncol. 2021; 12:1047–63.29. Rosner S, Liu C, Forde PM, Hu C. Association of pathologic complete response and long-term survival outcomes among patients treated with neoadjuvant chemotherapy or chemoradiotherapy for NSCLC: a meta-analysis. JTO Clin Res Rep. 2022; 3:100384.
Article30. Martinez-Meehan D, Lutfi W, Dhupar R, Christie N, Baker N, Schuchert M, et al. Factors associated with survival in complete pathologic response non-small cell lung cancer. Clin Lung Cancer. 2020; 21:349–56.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tumor Size Evaluation according to the T Component of the Seventh Edition of the International Association for the Study of Lung Cancer's TNM Classification: Interobserver Agreement between Radiologists and Computer-Aided Diagnosis System in Patients with Lung Cancer
- The Role of Neutrophil-to-Lymphocyte Ratio in Predicting Pathological Response for Resectable Non–Small Cell Lung Cancer Treated with Neoadjuvant Chemotherapy Combined with PD-1 Checkpoint Inhibitors
- Pathologic Evaluation of Breast Cancer after Neoadjuvant Therapy
- Clinical effects of neoafjuvant chemotherapy in stage IIIA non-small cell lung cancer
- Outcomes of the Initial Surgical Treatment without Neoadjuvant Therapy in Patients with Unexpected N2 Non-small Cell Lung Cancer