Cancer Res Treat.
2025 Apr;57(2):362-368. 10.4143/crt.2024.690.
Association between Tumor Size at the Time of Disease Progression and Survival Outcomes
- Affiliations
-
- 1Department of Medical Oncology and Hematology, Kyung Hee University Hospital, Kyung Hee University College of Medicine, Seoul, Korea
- 2Division of Hematology-Oncology, Department of Internal Medicine, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea
- 3Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 4Department of Internal Medicine, SMG-SNU Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea
- 5Department of Clinical Pharmacology and Therapeutics, Kyung Hee University Hospital, Seoul, Korea
- 6East-West Medical Research Institute, Kyung Hee University, Seoul, Korea
- 7Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- KMID: 2566855
- DOI: http://doi.org/10.4143/crt.2024.690
Abstract
- Purpose
This study evaluates the prognostic significance of tumor size at disease progression (PD) and depth of response (DOR) in cancer patients.
Materials and Methods
We performed post hoc analysis using data from six prospective clinical trials conducted by the Korean Cancer Study Group. Patients with tumor size at PD was categorized into ‘Mild PD’ and ‘Significant PD’ based on the cutoff values of relative change from baseline using maximally selected rank statistics. The overall survival (OS) and progression-free survival (PFS) were compared between PD and DOR categories.
Results
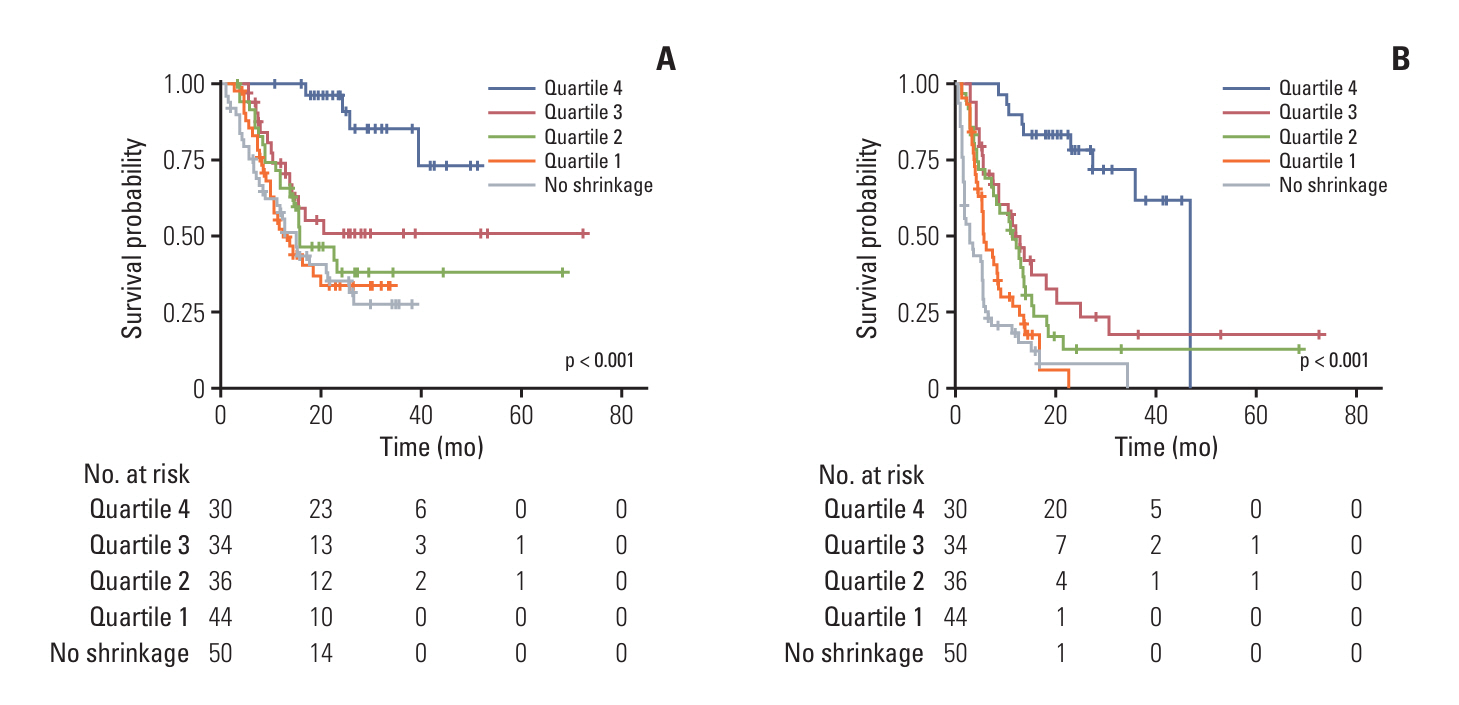
Among the 194 evaluable patients, 130 experienced PD. A 35.48% decrease from baseline in tumor size at PD was chosen for the cutoff between mild and significant PD for OS (mild PD: tumor size from the baseline ≤ −35.48%; significant PD > −35.48%). The mild PD had superior OS compared to the significant PD (25.8 vs. 12.8 months; Hazard ratio [HR] 0.47, 95% CI 0.266-0.843, p=0.009). When using an exploratory cutoff based on whether the tumor size was below vs. exceeded from the baseline (mild PD: tumor size from the baseline ≤ 0%; significant PD > 0%), OS remained significantly longer in the mild PD (17.1 vs. 11.8 months; HR 0.60, 95% CI 0.392-0.932, p=0.021). The greatest DOR was associated with the longest OS and PFS (p<0.001 for both).
Conclusion
Tumor size at PD and DOR were significant prognostic factors for progressive disease. Maintaining a sufficiently reduced tumor size even during PD was associated with better survival outcomes.
Keyword
Figure
Reference
-
References
1. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.
Article2. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–16.3. Sohaib A. Response assessment in daily practice: RECIST and its modifications. Cancer Imaging. 2014; 14:O35.
Article4. Lai GS, Li JR, Wang SS, Chen CS, Yang CK, Hung SC, et al. Tumor size significantly affects prognosis in pathological T3a renal cell carcinoma. Anticancer Res. 2022; 42:2185–91.
Article5. Feng H, Lyu Z, Zheng J, Zheng C, Wu Q, Liang W, et al. Association of tumor size with prognosis in colon cancer: a Surveillance, Epidemiology, and End Results (SEER) database analysis. Surgery. 2021; 169:1116–23.
Article6. Liu Y, He M, Zuo WJ, Hao S, Wang ZH, Shao ZM. Tumor size still impacts prognosis in breast cancer with extensive nodal involvement. Front Oncol. 2021; 11:585613.
Article7. Deng G, Ren JK, Wang HT, Deng L, Chen ZB, Fan YW, et al. Tumor burden score dictates prognosis of patients with combined hepatocellular cholangiocarcinoma undergoing hepatectomy. Front Oncol. 2022; 12:977111.
Article8. Matoba T, Minohara K, Kawakita D, Takano G, Oguri K, Murashima A, et al. Impact of tumor burden on survival in patients with recurrent or metastatic head and neck cancer treated with immune checkpoint inhibitors. Sci Rep. 2022; 12:14319.
Article9. Nicolo E, Tarantino P, D’Ecclesiis O, Antonarelli G, Boscolo Bielo L, Marra A, et al. Baseline tumor size as prognostic index in patients with advanced solid tumors receiving experimental targeted agents. Oncologist. 2024; 29:75–83.
Article10. Hwang S, Lee YJ, Kim KH, Ahn CS, Moon DB, Ha TY, et al. The impact of tumor size on long-term survival outcomes after resection of solitary hepatocellular carcinoma: single-institution experience with 2558 patients. J Gastrointest Surg. 2015; 19:1281–90.
Article11. Takeda FR, Ramos M, Pereira MA, Sallum RA, Ribeiro Junior U, Nahas SC, et al. Tumor size predicts worse prognosis in esophagogastric junction adenocarcinoma. Updates Surg. 2022; 74:1871–9.
Article12. Hothorn T, Lausen B. On the exact distribution of maximally selected rank statistics. Comput Stat Data Anal. 2003; 43:121–37.
Article13. Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007; 25:579–86.
Article14. McCoach CE, Blumenthal GM, Zhang L, Myers A, Tang S, Sridhara R, et al. Exploratory analysis of the association of depth of response and survival in patients with metastatic non-small-cell lung cancer treated with a targeted therapy or immunotherapy. Ann Oncol. 2017; 28:2707–14.
Article15. Talmadge JE. Clonal selection of metastasis within the life history of a tumor. Cancer Res. 2007; 67:11471–5.
Article16. Tilsed CM, Fisher SA, Nowak AK, Lake RA, Lesterhuis WJ. Cancer chemotherapy: insights into cellular and tumor microenvironmental mechanisms of action. Front Oncol. 2022; 12:960317.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Less than 60 Days Delay in Surgery on Tumor Progression and Survival Outcomes in Invasive Breast Cancer Patients
- The Expression of Apolipoprotein D in Hepatocellular Carcinoma
- Impact of tumor size on hepatectomy outcomes in hepatocellular carcinoma: a nationwide propensity score matching analysis
- Prognostic Factors of Atypical Meningioma: Overall Survival Rate and Progression Free Survival Rate
- Clinical Significance of Tumor Size in Patients with Hepatocellular Carcinoma