Cancer Res Treat.
2025 Apr;57(2):293-311. 10.4143/crt.2024.714.
The Era of Antibody Drug Conjugates in Lung Cancer: Trick or Threat?
- Affiliations
-
- 1Department of Pneumology, Hôpital Cochin APHP Centre, Paris, France
- 2Department of Cancer Medicine, Gustave Roussy Cancer Campus, Villejuif, France
- KMID: 2566850
- DOI: http://doi.org/10.4143/crt.2024.714
Abstract
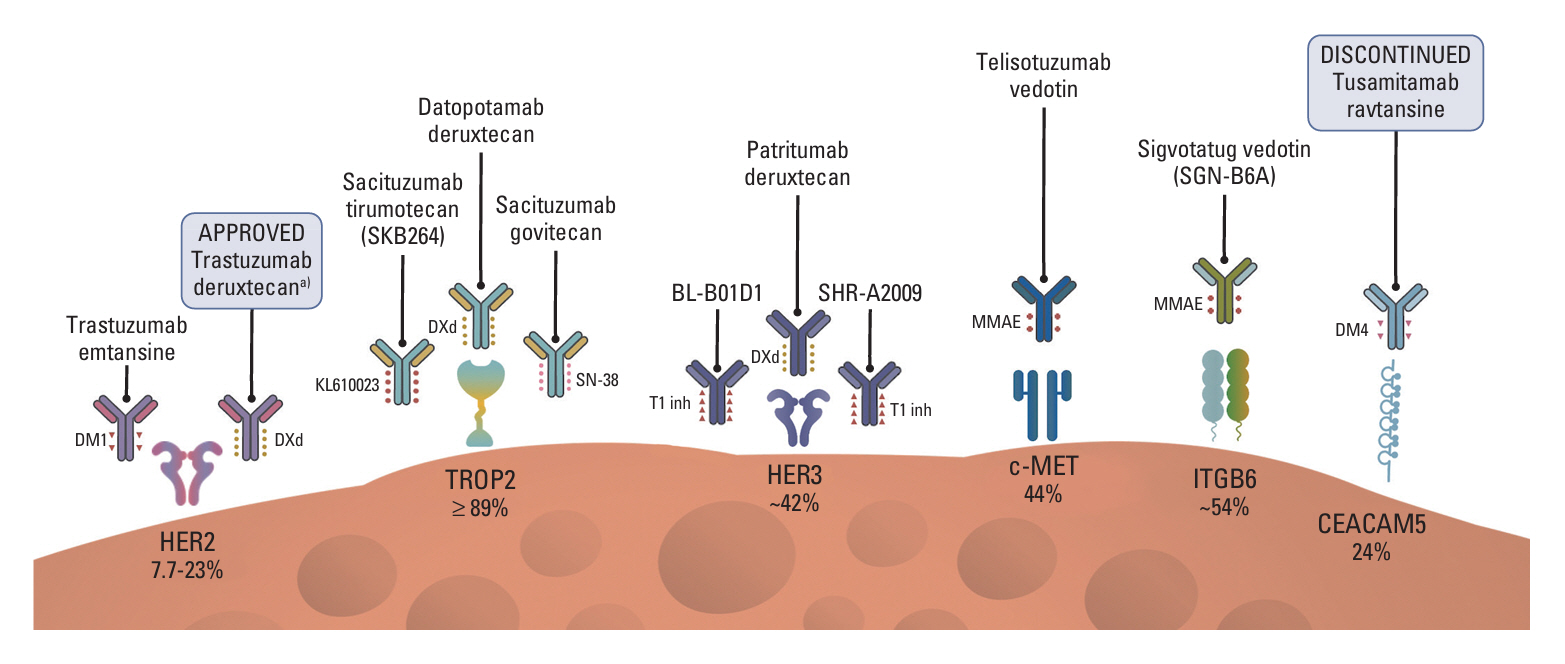
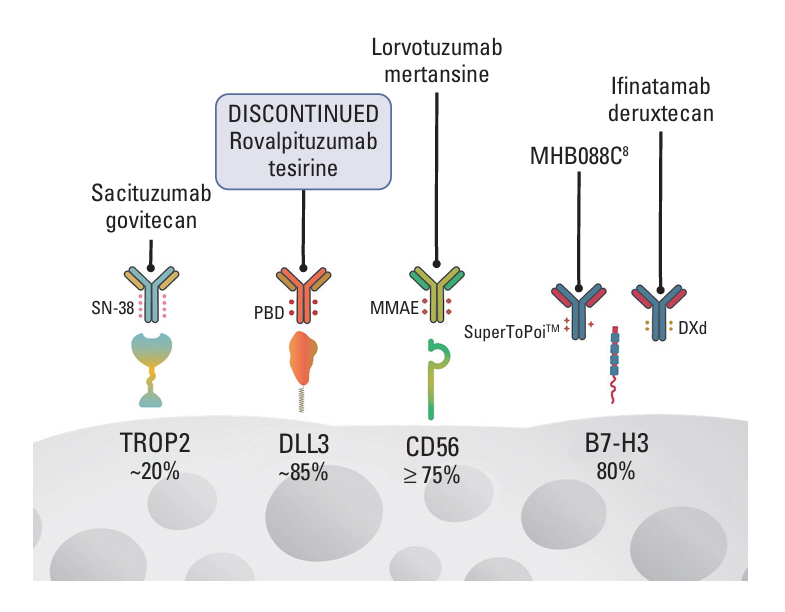
- Antibody drug conjugates (ADCs) are a novel class of therapeutics that structurally are composed by an antibody directed to a tumor epitope connected via a linker to a cytotoxic payload, and that have shown significant antitumor activity across a range of malignancies including lung cancer. In this article we review the pharmacology and design of ADCs, as well as we describe the results of different studies evaluating ADCs in lung cancer directed to several targets including HER2, HER3, TROP2, MET, CEACAM5 and DLL3.
Figure
Reference
-
References
1. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Pineros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019; 144:1941–53.
Article2. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. NCCN clinical practice guidelines in oncology (NCCN Guidelines) for non-small cell lung cancer (version 2.2021). National Comprehensive Cancer Network;2021.3. Fu Z, Li S, Han S, Shi C, Zhang Y. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct Target Ther. 2022; 7:93.
Article4. Li BT, Smit EF, Goto Y, Nakagawa K, Udagawa H, Mazieres J, et al. Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N Engl J Med. 2022; 386:241–51.
Article5. Levy B, Paz-Ares L, Rixe O, Su WC, Yang TY, Tolcher A, et al. MA13.07 TROPION-Lung02: initial results for datopotamab deruxtecan plus pembrolizumab and platinum chemotherapy in advanced NSCLC. J Thorac Oncol. 2022; 17(9 Suppl):S91.
Article6. Tiller KE, Tessier PM. Advances in antibody design. Annu Rev Biomed Eng. 2015; 17:191–216.
Article7. Yu J, Song Y, Tian W. How to select IgG subclasses in developing anti-tumor therapeutic antibodies. J Hematol Oncol. 2020; 13:45.
Article8. Drago JZ, Modi S, Chandarlapaty S. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat Rev Clin Oncol. 2021; 18:327–44.
Article9. de Goeij BE, Satijn D, Freitag CM, Wubbolts R, Bleeker WK, Khasanov A, et al. High turnover of tissue factor enables efficient intracellular delivery of antibody-drug conjugates. Mol Cancer Ther. 2015; 14:1130–40.
Article10. Jain N, Smith SW, Ghone S, Tomczuk B. Current ADC linker chemistry. Pharm Res. 2015; 32:3526–40.
Article11. Tsuchikama K, An Z. Antibody-drug conjugates: recent advances in conjugation and linker chemistries. Protein Cell. 2018; 9:33–46.
Article12. Bargh JD, Isidro-Llobet A, Parker JS, Spring DR. Cleavable linkers in antibody-drug conjugates. Chem Soc Rev. 2019; 48:4361–74.
Article13. Nolting B. Linker technologies for antibody-drug conjugates. In : Ducry L, editor. Antibody-drug conjugates. Methods in Molecular Biology, Vol. 1045. Humana Press;2013. p. 71–100.14. Tang H, Liu Y, Yu Z, Sun M, Lin L, Liu W, et al. The analysis of key factors related to ADCs structural design. Front Pharmacol. 2019; 10:373.
Article15. Zhao P, Zhang Y, Li W, Jeanty C, Xiang G, Dong Y. Recent advances of antibody drug conjugates for clinical applications. Acta Pharm Sin B. 2020; 10:1589–600.
Article16. Birrer MJ, Moore KN, Betella I, Bates RC. Antibody-drug conjugate-based therapeutics: state of the science. J Natl Cancer Inst. 2019; 111:538–49.
Article17. Nagayama A, Ellisen LW, Chabner B, Bardia A. Antibody-drug conjugates for the treatment of solid tumors: clinical experience and latest developments. Target Oncol. 2017; 12:719–39.
Article18. Hamblett KJ, Senter PD, Chace DF, Sun MM, Lenox J, Cerveny CG, et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin Cancer Res. 2004; 10:7063–70.
Article19. Hedrich WD, Fandy TE, Ashour HM, Wang H, Hassan HE. Antibody-drug conjugates: pharmacokinetic/pharmacodynamic modeling, preclinical characterization, clinical studies, and lessons learned. Clin Pharmacokinet. 2018; 57:687–703.
Article20. McCombs JR, Owen SC. Antibody drug conjugates: design and selection of linker, payload and conjugation chemistry. AAPS J. 2015; 17:339–51.
Article21. Kamath AV, Iyer S. Preclinical pharmacokinetic considerations for the development of antibody drug conjugates. Pharm Res. 2015; 32:3470–9.
Article22. Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008; 68:9280–90.
Article23. Hotta K, Aoe K, Kozuki T, Ohashi K, Ninomiya K, Ichihara E, et al. A phase II study of trastuzumab emtansine in HER2-positive non-small cell lung cancer. J Thorac Oncol. 2018; 13:273–9.
Article24. Li BT, Shen R, Buonocore D, Olah ZT, Ni A, Ginsberg MS, et al. Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: results from a phase II basket trial. J Clin Oncol. 2018; 36:2532–7.25. Li BT, Makker V, Buonocore DJ, Offin MD, Olah ZT, Panora E, et al. A multi-histology basket trial of ado-trastuzumab emtansine in patients with HER2 amplified cancers. J Clin Oncol. 2018; 36(15 Suppl):2502.
Article26. Li BT, Michelini F, Misale S, Cocco E, Baldino L, Cai Y, et al. HER2-mediated internalization of cytotoxic agents in ERBB2 amplified or mutant lung cancers. Cancer Discov. 2020; 10:674–87.27. Peters S, Stahel R, Bubendorf L, Bonomi P, Villegas A, Kowalski DM, et al. Trastuzumab emtansine (T-DM1) in patients with previously treated HER2-overexpressing metastatic non-small cell lung cancer: efficacy, safety, and biomarkers. Clin Cancer Res. 2019; 25:64–72.
Article28. Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer. 2006; 6:789–802.
Article29. Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 2016; 22:5097–108.30. Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016; 107:1039–46.31. Tsurutani J, Park H, Doi T, Modi S, Takahashi S, Nakagawa K, et al. OA02.07 Updated results of phase 1 study of DS-8201a in HER2-expressing or –mutated advanced non-small-cell lung cancer. J Thorac Oncol. 2018; 13(10 Suppl):S324.
Article32. Tsurutani J, Iwata H, Krop I, Janne PA, Doi T, Takahashi S, et al. Targeting HER2 with trastuzumab deruxtecan: a dose-expansion, phase I study in multiple advanced solid tumors. Cancer Discov. 2020; 10:688–701.
Article33. Smit EF, Nakagawa K, Nagasaka M, Felip E, Goto Y, Li BT, et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients with HER2-mutated metastatic non-small cell lung cancer (NSCLC): interim results of DESTINY-Lung01. J Clin Oncol. 2020; 38(15 Suppl):9504.
Article34. Nakagawa K, Nagasaka M, Felip E, Pacheco J, Baik C, Goto Y, et al. OA04.05 Trastuzumab deruxtecan in HER2-overexpressing metastatic non–small cell lung cancer: interim results of DESTINY-Lung01. J Thorac Oncol. 2021; 16(3 Suppl):S109–10.
Article35. Smit EF, Felip E, Uprety D, Nagasaka M, Nakagawa K, PazAres Rodriguez L, et al. Trastuzumab deruxtecan in patients with metastatic non-small-cell lung cancer (DESTINY-Lung01): primary results of the HER2-overexpressing cohorts from a single-arm, phase 2 trial. Lancet Oncol. 2024; 25:439–54.
Article36. Goto K, Goto Y, Kubo T, Ninomiya K, Kim SW, Planchard D, et al. Trastuzumab deruxtecan in patients with HER2-mutant metastatic non-small-cell lung cancer: primary results from the randomized, phase II DESTINY-Lung02 trial. J Clin Oncol. 2023; 41:4852–63.37. Trerotola M, Cantanelli P, Guerra E, Tripaldi R, Aloisi AL, Bonasera V, et al. Upregulation of Trop-2 quantitatively stimulates human cancer growth. Oncogene. 2013; 32:222–33.
Article38. Inamura K, Yokouchi Y, Kobayashi M, Ninomiya H, Sakakibara R, Subat S, et al. Association of tumor TROP2 expression with prognosis varies among lung cancer subtypes. Oncotarget. 2017; 8:28725–35.
Article39. Zeng P, Chen MB, Zhou LN, Tang M, Liu CY, Lu PH. Impact of TROP2 expression on prognosis in solid tumors: a systematic review and meta-analysis. Sci Rep. 2016; 6:33658.
Article40. Spira A, Lisberg A, Sands J, Greenberg J, Phillips P, Guevara F, et al. OA03.03 Datopotamab deruxtecan (Dato-DXd; DS-1062), a TROP2 ADC, in patients with advanced NSCLC: updated results of TROPION-PanTumor01 phase 1 study. J Thorac Oncol. 2021; 16(3 Suppl):S106–7.
Article41. Garon E, Johnson M, Lisberg A, Spira A, Yamamoto N, Heist R, et al. MA03.02 TROPION-PanTumor01: updated results from the NSCLC cohort of the phase 1 study of datopotamab deruxtecan in solid tumors. J Thorac Oncol. 2021; 16(10 Suppl):S892–3.
Article42. Shimizu T, Sands J, Yoh K, Spira A, Garon EB, Kitazono S, et al. First-in-human, phase I dose-escalation and dose-expansion study of trophoblast cell-surface antigen 2-directed antibody-drug conjugate datopotamab deruxtecan in non-small-cell lung cancer: TROPION-PanTumor01. J Clin Oncol. 2023; 41:4678–87.
Article43. Garon EB, Johnson ML, Lisberg AE, Spira A, Yamamoto N, Heist RS, et al. LBA49 Efficacy of datopotamab deruxtecan (Dato-DXd) in patients (pts) with advanced/metastatic (adv/met) non-small cell lung cancer (NSCLC) and actionable genomic alterations (AGAs): preliminary results from the phase I TROPION-PanTumor01 study. Ann Oncol. 2021; 32(Suppl 5):S1326–7.
Article44. Ahn MJ, Lisberg A, Paz-Ares L, Cornelissen R, Girard N, Pons-Tostivint E, et al. LBA12 Datopotamab deruxtecan (Dato-DXd) vs docetaxel in previously treated advanced/metastatic (adv/met) non-small cell lung cancer (NSCLC): results of the randomized phase III study TROPION-Lung01. Ann Oncol. 2023; 34(Suppl 2):S1305–6.45. Paz-Ares L, Ahn MJ, Lisberg AE, Kitazono S, Cho BC, Blumenschein G, et al. 1314MO TROPION-Lung05: datopotamab deruxtecan (Dato-DXd) in previously treated non-small cell lung cancer (NSCLC) with actionable genomic alterations (AGAs). Ann Oncol. 2023; 34(Suppl 2):S755–6.
Article46. Goto Y, Su WC, Levy BP, Rixe O, Yang TY, Tolcher AW, et al. TROPION-Lung02: datopotamab deruxtecan (Dato-DXd) plus pembrolizumab (pembro) with or without platinum chemotherapy (Pt-CT) in advanced non-small cell lung cancer (aNSCLC). J Clin Oncol. 2023; 41(16 Suppl):9004.
Article47. Planchard D, Cozic N, Wislez M, Chouaid C, Curcio H, Cousin S, et al. ICARUS-LUNG01: a phase 2 study of datopotomab deruxtecan (Dato-DXd) in patients with previously treated advanced non-small cell lung cancer (NSCLC), with sequential tissue biopsies and biomarkers analysis to predict treatment outcome. J Clin Oncol. 2024; 42(16 Suppl):8501.
Article48. Bardia A, Messersmith WA, Kio EA, Berlin JD, Vahdat L, Masters GA, et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann Oncol. 2021; 32:746–56.
Article49. Heist RS, Guarino MJ, Masters G, Purcell WT, Starodub AN, Horn L, et al. Therapy of advanced non-small-cell lung cancer with an SN-38-Anti-Trop-2 drug conjugate, sacituzumab govitecan. J Clin Oncol. 2017; 35:2790–7.
Article50. Paz-Ares LG, Juan-Vidal O, Mountzios GS, Felip E, Reinmuth N, de Marinis F, et al. Sacituzumab govitecan versus docetaxel for previously treated advanced or metastatic non-small cell lung cancer: the randomized, open-label phase III EVOKE-01 study. J Clin Oncol. 2024; 42:2860–72.51. Fang W, Cheng Y, Chen Z, Wang W, Li Y, Yin Y, et al. Abstract CT247: updated efficacy and safety of anti-TROP2 ADC SKB264 (MK-2870) for previously treated advanced NSCLC in phase 2 study. Cancer Res. 2024; 84(7 Suppl):CT247.
Article52. Scharpenseel H, Hanssen A, Loges S, Mohme M, Bernreuther C, Peine S, et al. EGFR and HER3 expression in circulating tumor cells and tumor tissue from non-small cell lung cancer patients. Sci Rep. 2019; 9:7406.
Article53. Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007; 316:1039–43.
Article54. Kawakami H, Yonesaka K. HER3 and its ligand, heregulin, as targets for cancer therapy. Recent Pat Anticancer Drug Discov. 2016; 11:267–74.
Article55. Li Q, Zhang R, Yan H, Zhao P, Wu L, Wang H, et al. Prognostic significance of HER3 in patients with malignant solid tumors. Oncotarget. 2017; 8:67140–51.
Article56. Lyu H, Han A, Polsdofer E, Liu S, Liu B. Understanding the biology of HER3 receptor as a therapeutic target in human cancer. Acta Pharm Sin B. 2018; 8:503–10.
Article57. Janne PA, Baik C, Su WC, Johnson ML, Hayashi H, Nishio M, et al. Efficacy and safety of patritumab deruxtecan (HER3-DXd) in EGFR inhibitor-resistant, EGFR-mutated non-small cell lung cancer. Cancer Discov. 2022; 12:74–89.58. Johnson ML, Steuer CE, Hayashi H, Su WC, Nishio M, Kim DW, et al. PP01.48 Efficacy and safety of patritumab deruxtecan (HER3-DXd) in locally advanced/metastatic non-small cell lung cancer (NSCLC) without EGFR-activating mutations. J Thorac Oncol. 2023; 18(3 Suppl):E30–1.
Article59. Yu HA, Goto Y, Hayashi H, Felip E, Chih-Hsin Yang J, Reck M, et al. HERTHENA-Lung01, a phase II trial of patritumab deruxtecan (HER3-DXd) in epidermal growth factor receptor-mutated non-small-cell lung cancer after epidermal growth factor receptor tyrosine kinase inhibitor therapy and platinum-based chemotherapy. J Clin Oncol. 2023; 41:5363–75.
Article60. Haikala HM, Lopez T, Kohler J, Eser PO, Xu M, Zeng Q, et al. EGFR inhibition enhances the cellular uptake and antitumor-activity of the HER3 antibody-drug conjugate HER3-DXd. Cancer Res. 2022; 82:130–41.
Article61. Zhang L, Ma Y, Zhao Y, Fang WF, Zhao H, Huang Y, et al. 1316MO BL-B01D1, a first-in-class EGFRxHER3 bispecific antibody-drug conjugate, in patients with non-small cell lung cancer: updated results from first-in-human phase I study. Ann Oncol. 2023; 34(Suppl 2):S758.
Article62. Zhou Q, Wu YL, Li J, Liu A, Cui J, Kuboki Y, et al. 658MO Phase I study of SHR-A2009, a HER3-targeted ADC, in advanced solid tumors. Ann Oncol. 2023; 34(Suppl 2):S463.
Article63. Zhou Q, Wu YL, Wu L, Zhang Y, Ni S, Liu A, et al. 642P Phase I study of SHR-A2009, a HER3-targeted ADC, in pretreated EGFR-mutated NSCLC. Ann Oncol. 2024; 35(Suppl 2):S509.
Article64. Awad MM, Oxnard GR, Jackman DM, Savukoski DO, Hall D, Shivdasani P, et al. MET exon 14 mutations in non-small-cell lung cancer are associated with advanced age and stage-dependent MET genomic amplification and c-Met overexpression. J Clin Oncol. 2016; 34:721–30.65. Ma PC, Tretiakova MS, MacKinnon AC, Ramnath N, Johnson C, Dietrich S, et al. Expression and mutational analysis of MET in human solid cancers. Genes Chromosomes Cancer. 2008; 47:1025–37.66. Schildhaus HU, Schultheis AM, Ruschoff J, Binot E, Merkelbach-Bruse S, Fassunke J, et al. MET amplification status in therapy-naive adeno- and squamous cell carcinomas of the lung. Clin Cancer Res. 2015; 21:907–15.67. Strickler JH, Weekes CD, Nemunaitis J, Ramanathan RK, Heist RS, Morgensztern D, et al. First-in-human phase I, dose-escalation and -expansion study of telisotuzumab vedotin, an antibody-drug conjugate targeting c-Met, in patients with advanced solid tumors. J Clin Oncol. 2018; 36:3298–306.
Article68. Waqar SN, Redman MW, Arnold SM, Hirsch FR, Mack PC, Schwartz LH, et al. A phase II study of telisotuzumab vedotin in patients with c-MET-positive stage IV or recurrent squamous cell lung cancer (LUNG-MAP Sub-study S1400K, NCT03574753). Clin Lung Cancer. 2021; 22:170–7.
Article69. Camidge DR, Bar J, Horinouchi H, Goldman JW, Moiseenko FV, Filippova E, et al. Telisotuzumab vedotin (Teliso-V) monotherapy in patients (pts) with previously treated c-Met–overexpressing (OE) advanced non-small cell lung cancer (NSCLC). J Clin Oncol. 2022; 40(16 Suppl):9016.
Article70. Camidge DR, Bar J, Horinouchi H, Goldman JW, Moiseenko FV, Filippova E, et al. Telisotuzumab vedotin monotherapy in patients with previously treated c-Met–overexpressing non-squamous EGFR wildtype advanced NSCLC: primary analysis of the LUMINOSITY trial. J Clin Oncol. 2024; 42(16 Suppl):103.
Article71. Camidge DR, Goldman J, Vasilopoulos A, Ansell P, Xia S, Bolotin E, et al. Abstract CT214: Preliminary efficacy of telisotuzumab vedotin (Teliso-V) treatment in the 2L/3L setting in MET gene amplified (MET Amp), c-Met protein overexpressing (c-Met OE), non-squamous, non-small cell lung cancer (NSQ NSCLC): retrospective analysis of LUMINOSITY. Cancer Res. 2023; 83(8 Suppl):CT214.72. Horinouchi H, Cho BC, Camidge DR, Goto K, Tomasini P, Li Y, et al. 515MO Phase Ib study of telisotuzumab vedotin (Teliso-V) and osimertinib in patients (Pts) with advanced EGFR-mutated (Mut), c-Met overexpressing (OE) non-small cell lung cancer (NSCLC): final efficacy and safety updates. Ann Oncol. 2023; 34(Suppl 4):S1670.
Article73. De Miguel M, Yamamoto N, Raimbourg J, Cho BC, Gottfried M, Stemmer SM, et al. 1257MO ABBV-400, a c-Met protein-targeting antibody-drug conjugate (ADC), in patients (Pts) with advanced EGFR wildtype (WT) non-squamous (NSQ) non-small cell lung cancer (NSCLC): results from a phase I study. Ann Oncol. 2024; 35(Suppl 2):S805–6.
Article74. Beauchemin N, Arabzadeh A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev. 2013; 32:643–71.
Article75. Zhang X, Han X, Zuo P, Zhang X, Xu H. CEACAM5 stimulates the progression of non-small-cell lung cancer by promoting cell proliferation and migration. J Int Med Res. 2020; 48:300060520959478.
Article76. Minton JP, Hoehn JL, Gerber DM, Horsley JS, Connolly DP, Salwan F, et al. Results of a 400-patient carcinoembryonic antigen second-look colorectal cancer study. Cancer. 1985; 55:1284–90.
Article77. Thompson JA, Grunert F, Zimmermann W. Carcinoembryonic antigen gene family: molecular biology and clinical perspectives. J Clin Lab Anal. 1991; 5:344–66.
Article78. Zhou J, Fan X, Chen N, Zhou F, Dong J, Nie Y, et al. Identification of CEACAM5 as a biomarker for prewarning and prognosis in gastric cancer. J Histochem Cytochem. 2015; 63:922–30.
Article79. Powell E, Shao J, Picon HM, Bristow C, Ge Z, Peoples M, et al. A functional genomic screen in vivo identifies CEACAM5 as a clinically relevant driver of breast cancer metastasis. NPJ Breast Cancer. 2018; 4:9.
Article80. Hammarstrom S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999; 9:67–81.
Article81. Gazzah A, Bedard PL, Hierro C, Kang YK, Abdul Razak A, Ryu MH, et al. Safety, pharmacokinetics, and antitumor activity of the anti-CEACAM5-DM4 antibody-drug conjugate tusamitamab ravtansine (SAR408701) in patients with advanced solid tumors: first-in-human dose-escalation study. Ann Oncol. 2022; 33:416–25.
Article82. Gazzah A, Cousin S, Boni V, Ricordel C, Kim TM, Kim JS, et al. First-in-human phase 1 study of the antibody-drug conjugate (ADC) SAR408701 in advanced solid tumors: dose-expansion cohort of patients (pts) with non-squamous non-small cell lung cancer (NSQ NSCLC). J Clin Oncol. 2019; 37(15 Suppl):9072.
Article83. Ricordel C, Barlesi F, Cousin S, Cho BC, Calvo E, Kim TM, et al. Safety and efficacy of tusamitamab ravtansine (SAR408701) in long-term treated patients with nonsquamous non–small cell lung cancer (NSQ NSCLC) expressing carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5). J Clin Oncol. 2022; 40(16 Suppl):9039.
Article84. Li F, Shang Y, Shi F, Zhang L, Yan J, Sun Q, et al. Expression of integrin beta6 and HAX-1 correlates with aggressive features and poor prognosis in esophageal squamous cell carcinoma. Cancer Manag Res. 2020; 12:9599–608.85. Elayadi AN, Samli KN, Prudkin L, Liu YH, Bian A, Xie XJ, et al. A peptide selected by biopanning identifies the integrin alphavbeta6 as a prognostic biomarker for non-small cell lung cancer. Cancer Res. 2007; 67:5889–95.86. Lyon RP, Jonas M, Frantz C, Trueblood ES, Yumul R, Westendorf L, et al. SGN-B6A: a new vedotin antibody-drug conjugate directed to integrin beta-6 for multiple carcinoma indications. Mol Cancer Ther. 2023; 22:1444–53.
Article87. Peters S, Hollebecque A, Sehgal K, Lopez JS, Calvo E, Dowlati A, et al. Efficacy and safety of sigvotatug vedotin, an investigational ADC, in NSCLC: updated phase 1 results (SGNB6A-001). J Clin Oncol. 2024; 42(16 Suppl):8521.
Article88. Saunders LR, Bankovich AJ, Anderson WC, Aujay MA, Bheddah S, Black K, et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med. 2015; 7:302ra136.
Article89. Blackhall F, Jao K, Greillier L, Cho BC, Penkov K, Reguart N, et al. Efficacy and safety of rovalpituzumab tesirine compared with topotecan as second-line therapy in DLL3-high SCLC: results from the phase 3 TAHOE study. J Thorac Oncol. 2021; 16:1547–58.
Article90. Johnson ML, Zvirbule Z, Laktionov K, Helland A, Cho BC, Gutierrez V, et al. Rovalpituzumab tesirine as a maintenance therapy after first-line platinum-based chemotherapy in patients with extensive-stage-SCLC: results from the phase 3 MERU study. J Thorac Oncol. 2021; 16:1570–81.
Article91. Socinski MA, Kaye FJ, Spigel DR, Kudrik FJ, Ponce S, Ellis PM, et al. Phase 1/2 study of the CD56-targeting antibody-drug conjugate lorvotuzumab mertansine (IMGN901) in combination with carboplatin/etoposide in small-cell lung cancer patients with extensive-stage disease. Clin Lung Cancer. 2017; 18:68–76.
Article92. Gray JE, Heist RS, Starodub AN, Camidge DR, Kio EA, Masters GA, et al. Therapy of small cell lung cancer (SCLC) with a topoisomerase-I-inhibiting antibody-drug conjugate (ADC) targeting Trop-2, sacituzumab govitecan. Clin Cancer Res. 2017; 23:5711–9.
Article93. Dowlati A, Cervantes A, Babu S, Hamilton EP, Wong SF, Tazbirkova A, et al. 1990MO Sacituzumab govitecan (SG) as second-line (2L) treatment for extensive stage small cell lung cancer (ES-SCLC): preliminary results from the phase II TROPiCS-03 basket trial. Ann Oncol. 2023; 34(Suppl 2):S1061–2.
Article94. Doi T, Patel M, Falchook GS, Koyama T, Friedman CF, PihaPaul S, et al. 453O DS-7300 (B7-H3 DXd antibody-drug conjugate [ADC]) shows durable antitumor activity in advanced solid tumors: extended follow-up of a phase I/II study. Ann Oncol. 2022; 33(Suppl 7):S744–5.
Article95. Shen L, Zhou C, Meng X, Sun Y, Ji Y, Yang H, et al. Results of a phase 1/2 study of MHB088C: a novel B7H3 antibody-drug conjugate (ADC) incorporating a potent DNA topoisomerase I inhibitor in recurrent or metastatic solid tumors. J Clin Oncol. 2024; 42(16 Suppl):3012.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Review of Antibody-Drug Conjugates
- Strategies and Advancement in Antibody-Drug Conjugate Optimization for Targeted Cancer Therapeutics
- The Development of Antibody-Drug Conjugates for Urothelial Carcinoma Treatment
- Antibody-Drug Conjugates in Head and Neck Cancer
- Recent breakthroughs in the management of locally advanced and recurrent/metastatic cervical cancer