Kosin Med J.
2024 Dec;39(4):272-280. 10.7180/kmj.24.155.
Comparative analysis of Access PCT and Elecsys BRAHMS PCT assays for procalcitonin measurements
- Affiliations
-
- 1Department of Laboratory Medicine, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea
- 2Department of Psychiatry, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea
- KMID: 2562811
- DOI: http://doi.org/10.7180/kmj.24.155
Abstract
- Background
Procalcitonin (PCT) is a crucial biomarker for diagnosing sepsis and managing antibiotic therapy. This study evaluated the analytical performance and comparability of the Access PCT and Elecsys BRAHMS PCT assays.
Methods
The precision, detection capability, linearity, and reference range of both assays were assessed. A comparative analysis included 182 patient samples categorized into four risk groups to compare the results between Access PCT and Elecsys BRAHMS PCT assays.
Results
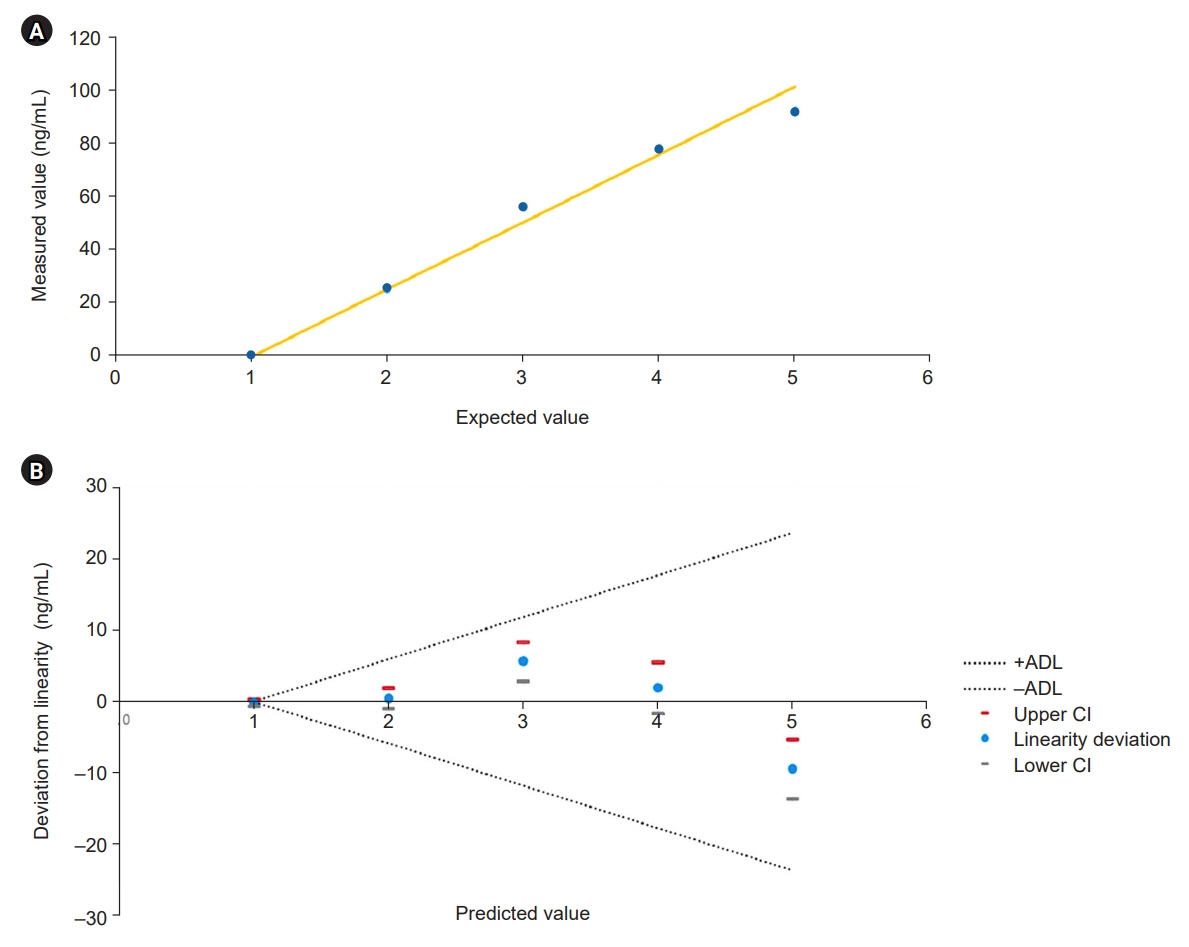
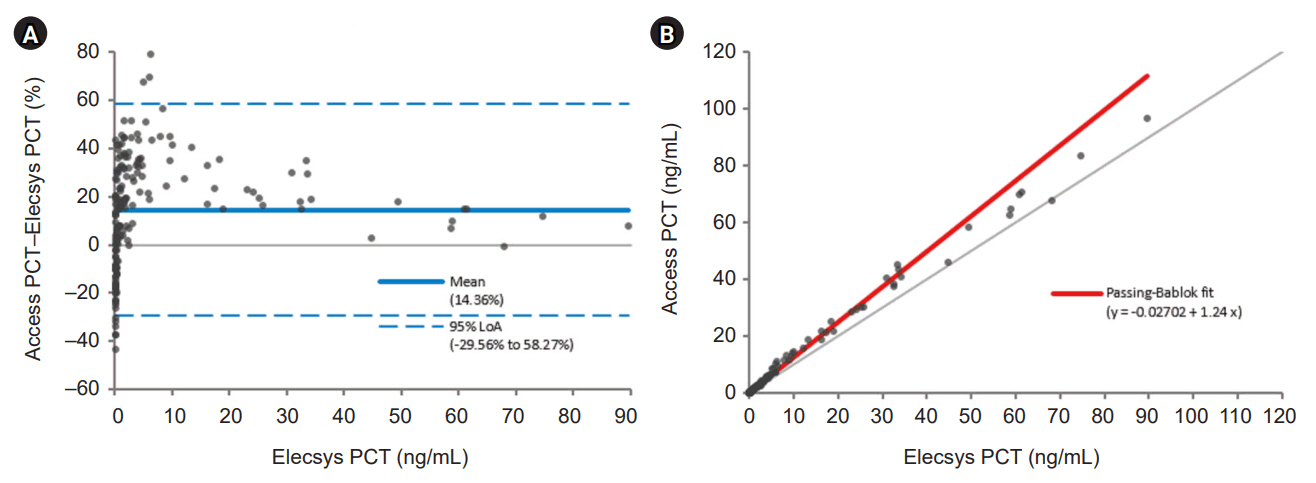
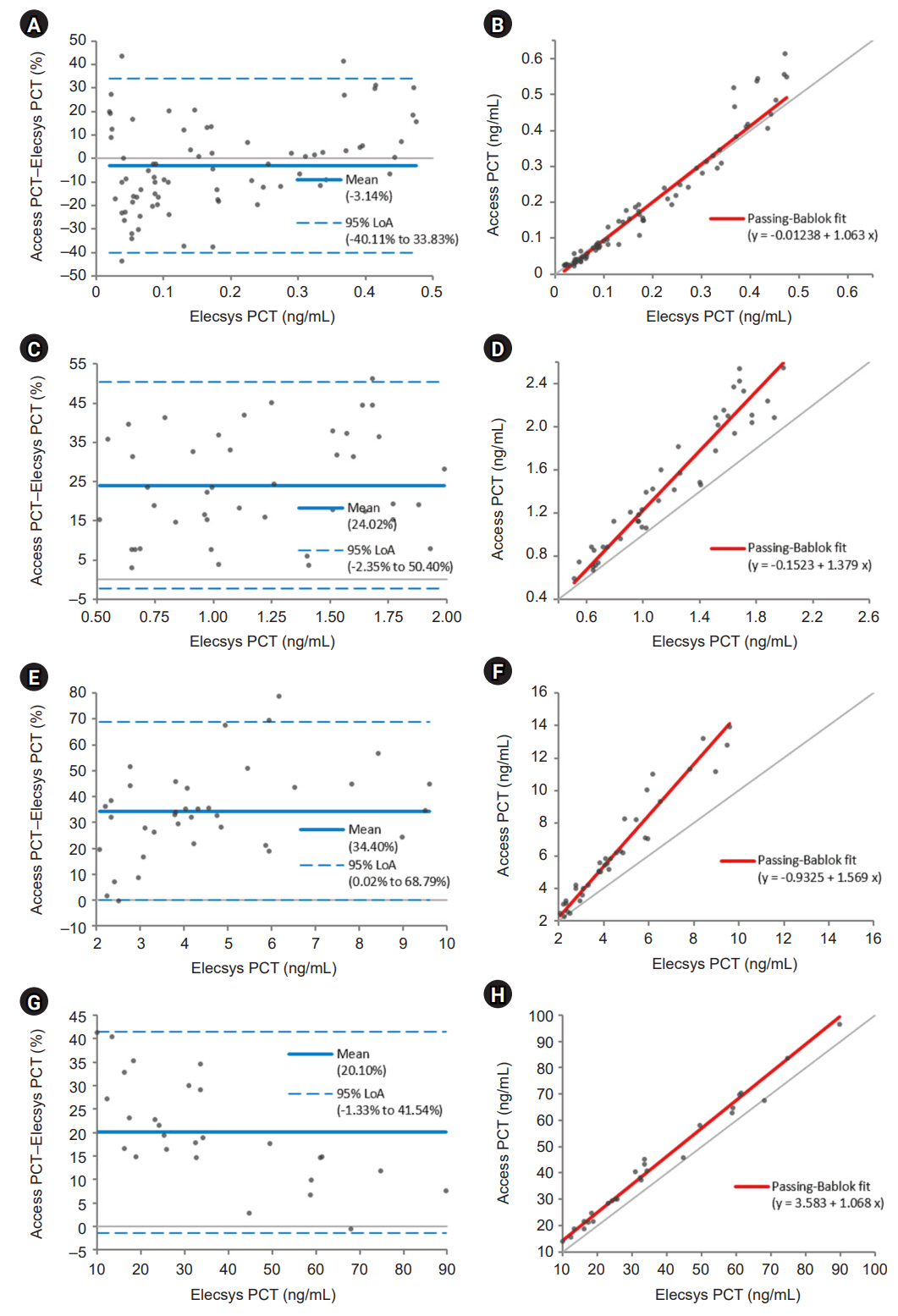
The Access PCT assay demonstrated precision within the manufacturer’s threshold, and its detection capabilities were verified. This assay exhibited excellent linearity and appropriate reference intervals. Comparative analysis indicated that the Access PCT assay reported higher overall PCT levels than the Elecsys BRAHMS assay, with high agreement between the assays (κ=0.941). However, the biases varied across different PCT concentration intervals.
Conclusions
Both the Access PCT and Elecsys BRAHMS PCT assays performed robustly with notable concordance but varying biases at different concentration intervals. The observed biases require careful consideration in clinical decision-making, especially when adopting novel assay systems. Standardizing the calibration across different platforms is recommended to improve assay comparability.
Keyword
Figure
Reference
-
References
1. Hamade B, Huang DT. Procalcitonin: where are we now? Crit Care Clin. 2020; 36:23–40.2. Samsudin I, Vasikaran SD. Clinical utility and measurement of procalcitonin. Clin Biochem Rev. 2017; 38:59–68.3. Lippi G, Salvagno GL, Gelati M, Pucci M, Demonte D, Faggian D, et al. Analytical evaluation of the new Beckman Coulter access procalcitonin (PCT) chemiluminescent immunoassay. Diagnostics (Basel). 2020; 10:128.
Article4. Dipalo M, Guido L, Micca G, Pittalis S, Locatelli M, Motta A, et al. Multicenter comparison of automated procalcitonin immunoassays. Pract Lab Med. 2015; 2:22–8.
Article5. Dupuy AM, Bargnoux AS, Larcher R, Merindol A, Masetto T, Badiou S, et al. Bioanalytical performance of a new particle-enhanced method for measuring procalcitonin. Diagnostics (Basel). 2020; 10:461.
Article6. Dupuy AM, Ruffel L, Bargnoux AS, Badiou S, Cristol JP. Analytical evaluation of the performances of a new procalcitonin immunoassay. Clin Chem Lab Med. 2021; 60:77–80.
Article7. Chambliss AB, Hayden J, Colby JM. Evaluation of procalcitonin immunoassay concordance near clinical decision points. Clin Chem Lab Med. 2019; 57:1414–21.
Article8. Huynh HH, Boeuf A, Pfannkuche J, Schuetz P, Thelen M, Nordin G, et al. Harmonization status of procalcitonin measurements: what do comparison studies and EQA schemes tell us? Clin Chem Lab Med. 2021; 59:1610–22.
Article9. Lippi G, Salvagno GL, Gelati M, Pucci M, Lo Cascio C, Demonte D, et al. Two-center comparison of 10 fully-automated commercial procalcitonin (PCT) immunoassays. Clin Chem Lab Med. 2019; 58:77–84.
Article10. CLSI. User verification of precision and estimation of bias: approved guideline. 3rd ed. Clinical and Laboratory Standards Institute;2014.11. Johnson R. Assessment of bias with emphasis on method comparison. Clin Biochem Rev. 2008; 29 Suppl 1(Suppl 1):S37–42.12. Aguirre JJ, Ness K, Algeciras-Schimnich A. Application of the CLSI EP15-A3 guideline as an alternative troubleshooting tool for verification of assay precision. Am J Clin Pathol. 2019; 152(Suppl 1):S88.
Article13. Jeong S, Hong YR, Hwang H. Performance comparison between Elecsys Anti-SARS-CoV-2 and Anti-SARS-CoV-2 S and Atellica IM SARS-CoV-2 Total and SARS-CoV-2 IgG assays. Kosin Med J. 2022; 37:154–62.
Article14. Clinical and Laboratory Standards Institute (CLSI). User protocol for evaluation of qualitative test performance: approved guideline. 2nd ed. CLSI;2008.15. Cho J. Evaluating and establishing the linearity interval and extended measuring interval. Lab Med Online. 2024; 14:163–75.
Article16. Badakhshan SN, Ghazizadeh H, Mohammadi-Bajgiran M, Esmaily H, Khorasani MY, Bohn MK, et al. Age-specific reference intervals for liver function tests in healthy neonates, infants, and young children in Iran. J Clin Lab Anal. 2023; 37:e24995.
Article17. Broughton PM. Carry-over in automatic analysers. J Automat Chem. 1984; 6:94–5.
Article18. Schuetz P. How to best use procalcitonin to diagnose infections and manage antibiotic treatment. Clin Chem Lab Med. 2022; 61:822–8.
Article19. Heo W, Park HD. Analytical and clinical performance of the Advansure i3 procalcitonin assay. Scand J Clin Lab Invest. 2021; 81:546–51.
Article20. Cho HW, Kim SH, Cho Y, Jeong SH, Lee SG. Concordance of three automated procalcitonin immunoassays at medical decision points. Ann Lab Med. 2021; 41:419–23.
Article21. Choi H, Tashpulatova Z, Moon SY, Choi J, Kim JY, Lee SM. Evaluation of the Beckman Coulter access procalcitonin assay: analytical and clinical performance. Clin Chem Lab Med. 2021; 60:e50–3.
Article22. Eidizadeh A, Wiederhold M, Schnelle M, Binder L. Comparison of a novel automated DiaSys procalcitonin immunoassay with four different BRAHMS-partnered immunoassays. Pract Lab Med. 2022; 30:e00274.
Article23. Meisner M. Procalcitonin: biochemistry and clinical diagnosis. Uni-Med Verlag AG;2010.24. Chambliss AB, Patel K, Colon-Franco JM, Hayden J, Katz SE, Minejima E, et al. AACC guidance document on the clinical use of procalcitonin. J Appl Lab Med. 2023; 8:598–634.
Article25. Farooq A, Colon-Franco JM. Procalcitonin and its limitations: why a biomarker's best isn't good enough. J Appl Lab Med. 2019; 3:716–9.
Article26. Hall MK, Kea B, Wang R. Recognizing bias in studies of diagnostic tests part 1: patient selection. Emerg Med J. 2019; 36:431–4.
Article27. Lorde N, Elgharably A, Kalaria T. Impact of variation between assays and reference intervals in the diagnosis of endocrine disorders. Diagnostics (Basel). 2023; 13:3453.
Article28. Huynh HH, Boeuf A, Vinh J, Delatour V; IFCC Working Group on Standardization of Procalcitonin assays (WG-PCT). Evaluation of the necessity and the feasibility of the standardization of procalcitonin measurements: activities of IFCC WG-PCT with involvement of all stakeholders. Clin Chim Acta. 2021; 515:111–21.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Utility of Procalcitonin on Antibiotic Stewardship: A Narrative Review
- Procalcitonin as a Diagnostic Marker for Bacteremia in Terminal Cancer Patients
- Update on Procalcitonin Measurements
- Clinical Availability of Serum Procalcitonin Level in the Diagnosis of Neonatal Bacterial Infection
- Evaluation of the Clinical Performance of an Automated Procalcitonin Assay for the Quantitative Detection of Bloodstream Infection