Ann Hepatobiliary Pancreat Surg.
2024 Nov;28(4):440-450. 10.14701/ahbps.24-110.
Construction and validation of a preoperative prognostic model integrating the novel aspartate aminotransferasealbumin score for hepatocellular carcinoma patients undergoing liver resection
- Affiliations
-
- 1Department of Surgery, Meiwa Hospital, Hyogo, Japan
- KMID: 2561576
- DOI: http://doi.org/10.14701/ahbps.24-110
Abstract
- Backgrounds/Aims
Patients undergoing liver resection for hepatocellular carcinoma (HCC) often possess good liver reserve, which may limit the prognostic effectiveness of existing liver function scores. This study aimed to develop a novel liver function score and a preoperative prognostic model specifically for HCC resection patients.
Methods
Eight hundred twenty-seven HCC patients undergoing initial liver resection were segregated into training and validation cohorts in a 6:4 ratio. Cox regression analysis was employed to identify significant parameters influencing overall survival. The efficacy of the liver function score and prognostic model was evaluated using metrics such as the area under the receiver operating characteristic curve.
Results
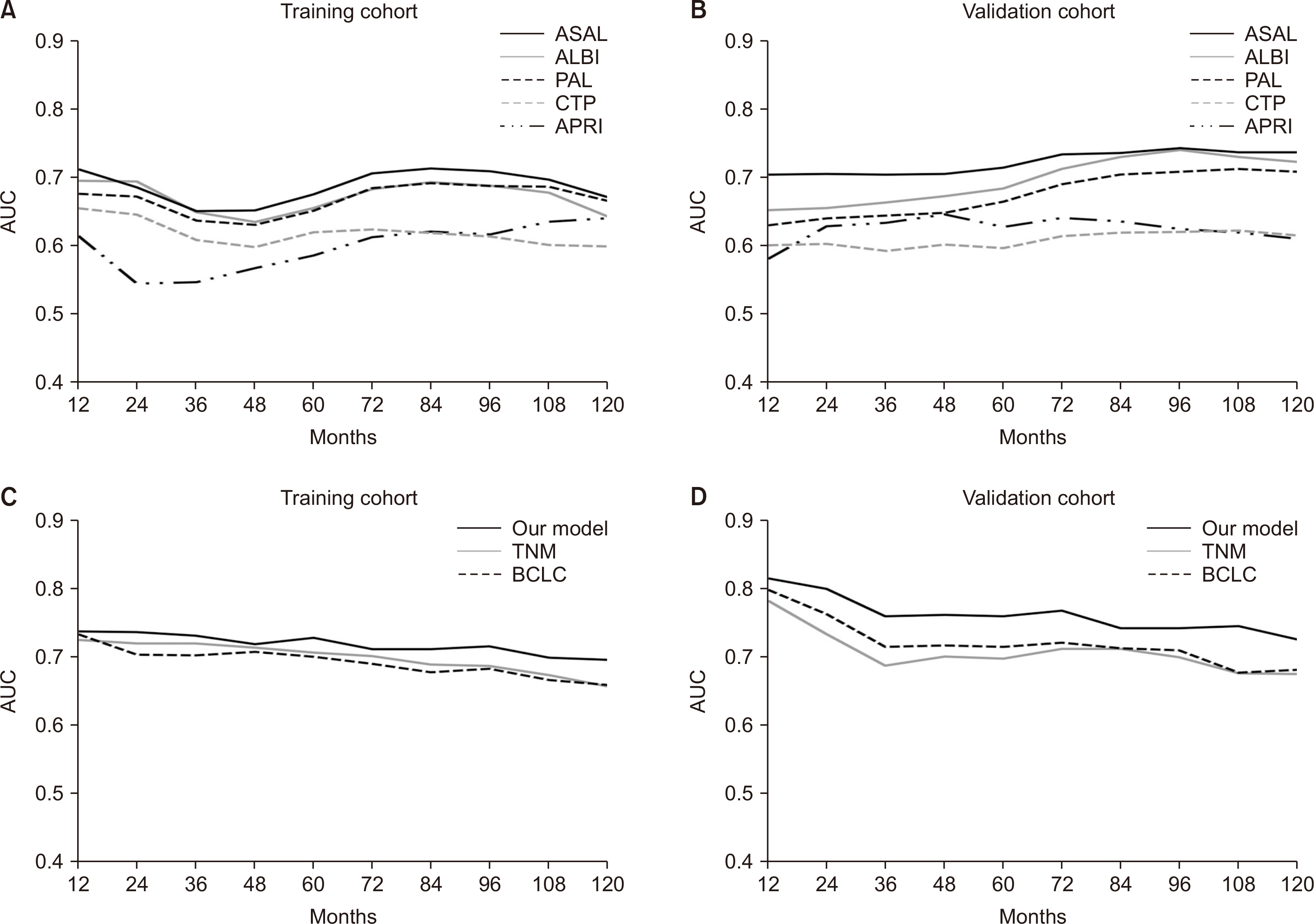
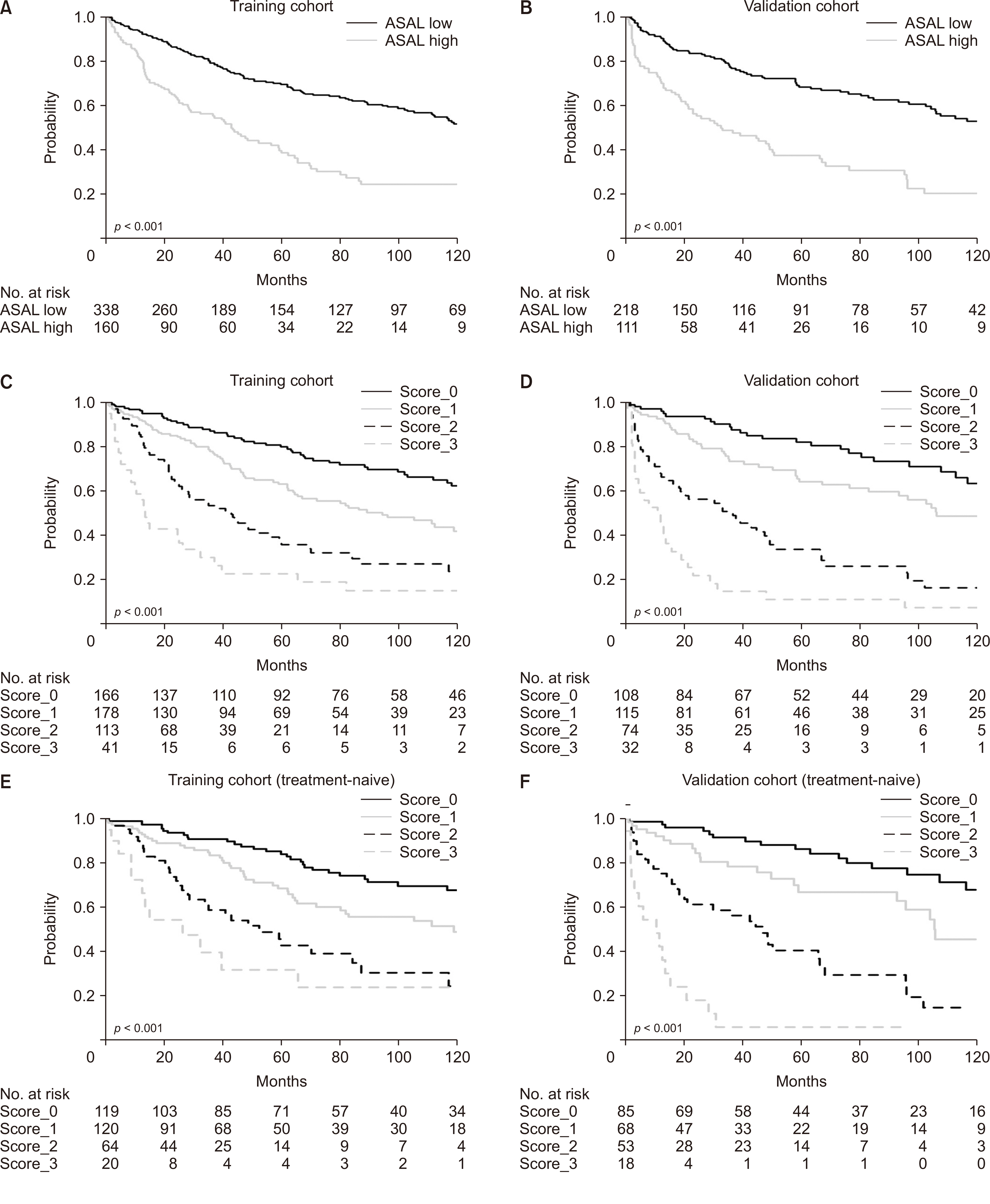
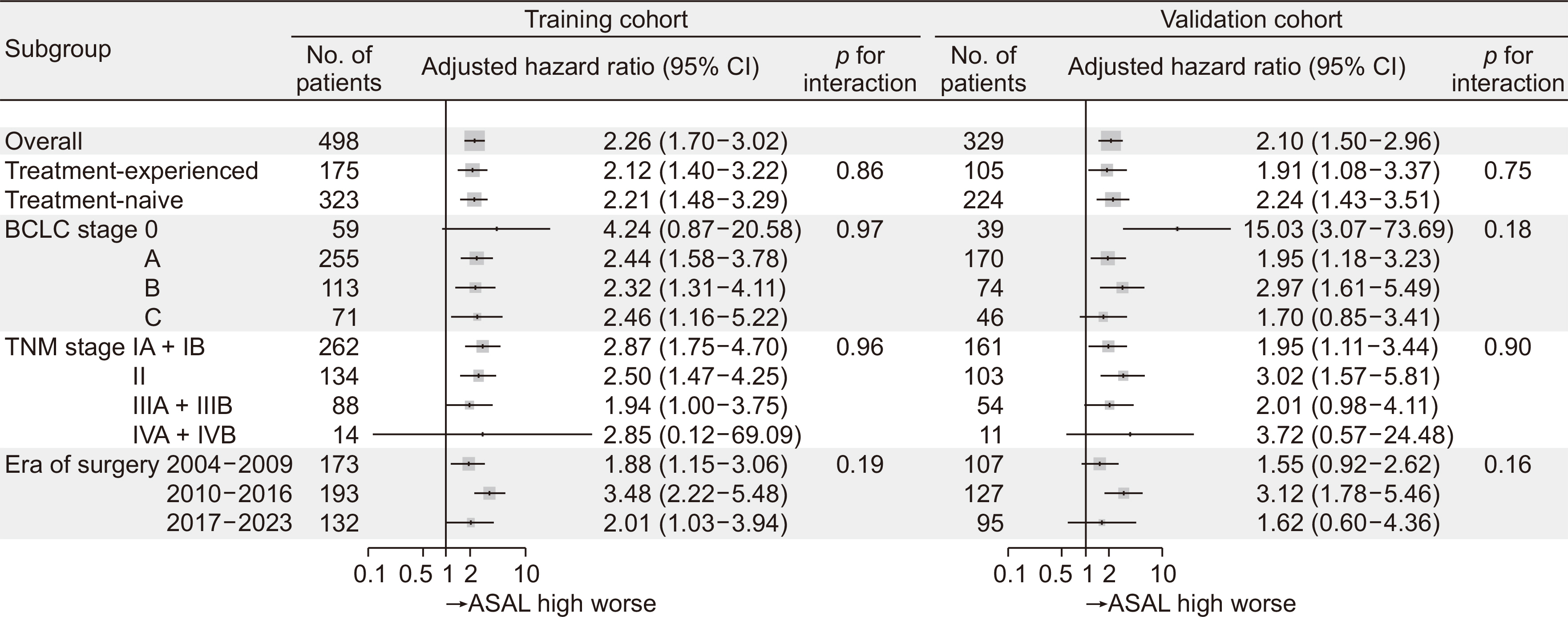
Aspartate aminotransferase (AST) and albumin emerged as significant prognostic indicators. The AST-albumin (ASAL) score, calculated as exp [AST (IU/L) × 0.005 – albumin (g/dL) × 1.043] × 100, outperformed existing scores such as Child-TurcottePugh, albumin-bilirubin, platelet-albumin, and AST-platelet ratio index in both training and validation cohorts. Additionally, a scoring model that combined the ASAL score with alpha-fetoprotein and the up-to-seven criterion exhibited superior discriminatory capabilities compared to the American Joint Committee on Cancer tumor, node, metastasis stage, and Barcelona Clinic Liver Cancer stage.
Conclusions
The proposed prognostic model that integrates the novel ASAL score offers promising prognostic potential for HCC patients undergoing liver resection.
Keyword
Figure
Reference
-
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–249. DOI: 10.3322/caac.21660. PMID: 33538338.2. Shah SA, Cleary SP, Wei AC, Yang I, Taylor BR, Hemming AW, et al. 2007; Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery. 141:330–339. DOI: 10.1016/j.surg.2006.06.028. PMID: 17349844.3. Lu LC, Cheng AL, Poon RT. 2014; Recent advances in the prevention of hepatocellular carcinoma recurrence. Semin Liver Dis. 34:427–434. DOI: 10.1055/s-0034-1394141. PMID: 25369304.4. Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. 2015; Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 33:550–558. DOI: 10.1200/JCO.2014.57.9151. PMID: 25512453. PMCID: PMC4322258.5. Miyata T, Yamashita YI, Arima K, Higashi T, Hayashi H, Imai K, et al. 2021; Alteration of prognostic efficacy of albumin-bilirubin grade and Child-Pugh score according to liver fibrosis in hepatocellular carcinoma patients with Child-Pugh A following hepatectomy. Ann Gastroenterol Surg. 6:127–134. DOI: 10.1002/ags3.12498. PMID: 35106423. PMCID: PMC8786693.6. Peng Y, Wei Q, He Y, Xie Q, Liang Y, Zhang L, et al. 2020; ALBI versus Child-Pugh in predicting outcome of patients with HCC: a systematic review. Expert Rev Gastroenterol Hepatol. 14:383–400. DOI: 10.1080/17474124.2020.1748010. PMID: 32240595.7. Shindoh J, Kawamura Y, Kobayashi Y, Kiya Y, Sugawara T, Akuta N, et al. 2019; Platelet-albumin score as a sensitive measure for surgical risk prediction and survival outcomes of patients with hepatocellular carcinoma. J Gastrointest Surg. 23:76–83. DOI: 10.1007/s11605-018-3871-1. PMID: 30022441.8. Le Roy B, Grégoire E, Cossé C, Serji B, Golse N, Adam R, et al. 2018; Indocyanine green retention rates at 15 min predicted hepatic decompensation in a Western population. World J Surg. 42:2570–2578. DOI: 10.1007/s00268-018-4464-6. PMID: 29340728.9. Chun YS, Pawlik TM, Vauthey JN. 2018; 8th edition of the AJCC cancer staging manual: pancreas and hepatobiliary cancers. Ann Surg Oncol. 25:845–847. DOI: 10.1245/s10434-017-6025-x. PMID: 28752469.10. The Cancer of the Liver Italian Program (Clip) Investigators. 1998; A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 28:751–755.11. Kudo M, Chung H, Osaki Y. 2003; Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol. 38:207–215. DOI: 10.1007/s005350300038. PMID: 12673442.12. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. 2022; BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 76:681–693. DOI: 10.1016/j.jhep.2021.11.018. PMID: 34801630. PMCID: PMC8866082.13. Brozzetti S, D'Alterio C, Bini S, Antimi J, Rocco B, Fassari A, et al. 2022; Surgical resection is superior to TACE in the treatment of HCC in a well selected cohort of BCLC-B elderly patients-a retrospective observational study. Cancers (Basel). 14:4422. DOI: 10.3390/cancers14184422. PMID: 36139581. PMCID: PMC9496726.14. Wee IJY, Moe FNN, Sultana R, Ang RWT, Quek PPS, Goh BKP, et al. 2022; Extending surgical resection for hepatocellular carcinoma beyond Barcelona Clinic for Liver Cancer (BCLC) stage A: a novel application of the modified BCLC staging system. J Hepatocell Carcinoma. 9:839–851. DOI: 10.2147/JHC.S370212. PMID: 35999856. PMCID: PMC9393033.15. Huang J, Yang Y, Xia Y, Liu FC, Liu L, Zhu P, et al. 2021; Prediction of patient survival following hepatic resection in early-stage hepatocellular carcinoma with indexed ratios of aspartate aminotransferase to platelets: a retrospective cohort study. Cancer Manag Res. 13:1733–1746. DOI: 10.2147/CMAR.S284950. PMID: 33642875. PMCID: PMC7903956.16. Deng W, Chen F, Li Y, Xu L. 2023; Development of a clinical scoring model to predict the overall and relapse-free survival of patients with hepatocellular carcinoma following a hepatectomy. Mol Clin Oncol. 19:87. DOI: 10.3892/mco.2023.2683. PMID: 37854326. PMCID: PMC10580259.17. Lu Y, Ren S, Jiang J. 2023; Development and validation of a nomogram for survival prediction in hepatocellular carcinoma after partial hepatectomy. BMC Surg. 23:27. DOI: 10.1186/s12893-023-01922-x. PMID: 36717904. PMCID: PMC9885608.18. Ishizuka M, Kubota K, Kita J, Shimoda M, Kato M, Mori S, et al. 2015; Aspartate aminotransferase-to-platelet ratio index is associated with liver cirrhosis in patients undergoing surgery for hepatocellular carcinoma. J Surg Res. 194:63–68. DOI: 10.1016/j.jss.2014.09.009. PMID: 25291961.19. Newson RB. 2010; Comparing the predictive powers of survival models using Harrell's C or Somers' D. Stata J. 10:339–358. DOI: 10.1177/1536867X1001000303.20. Peng W, Shen J, Dai J, Leng S, Xie F, Zhang Y, et al. 2022; Preoperative aspartate aminotransferase to albumin ratio correlates with tumor characteristics and predicts outcome of hepatocellular carcinoma patients after curative hepatectomy: a multicenter study. BMC Surg. 22:307. DOI: 10.1186/s12893-022-01751-4. PMID: 35945520. PMCID: PMC9364544.21. Wang F, Gao S, Wu M, Zhao D, Sun H, Yav S, et al. 2023; The prognostic role of the AST/ALT ratio in hepatocellular carcinoma patients receiving thermal ablation combined with simultaneous TACE. BMC Gastroenterol. 23:80. DOI: 10.1186/s12876-023-02719-1. PMID: 36944920. PMCID: PMC10029314.22. Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, et al. 2002; Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 122:366–375. DOI: 10.1053/gast.2002.30983. PMID: 11832451.23. Dang CV. 2012; Links between metabolism and cancer. Genes Dev. 26:877–890. DOI: 10.1101/gad.189365.112. PMID: 22549953. PMCID: PMC3347786.24. Sookoian S, Pirola CJ. 2015; Liver enzymes, metabolomics and genome-wide association studies: from systems biology to the personalized medicine. World J Gastroenterol. 21:711–725. DOI: 10.3748/wjg.v21.i3.711. PMID: 25624707. PMCID: PMC4299326.25. Kamimoto Y, Horiuchi S, Tanase S, Morino Y. 1985; Plasma clearance of intravenously injected aspartate aminotransferase isozymes: evidence for preferential uptake by sinusoidal liver cells. Hepatology. 5:367–375. DOI: 10.1002/hep.1840050305. PMID: 3997068.26. Witjes CD, IJzermans JN, van der Eijk AA, Hansen BE, Verhoef C, de Man RA. 2011; Quantitative HBV DNA and AST are strong predictors for survival after HCC detection in chronic HBV patients. Neth J Med. 69:508–513. DOI: 10.1016/S0168-8278(11)61027-1.27. Takeishi K, Maeda T, Shirabe K, Tsujita E, Yamashita Y, Harimoto N, et al. 2015; Clinicopathologic features and outcomes of non-B, non-C hepatocellular carcinoma after hepatectomy. Ann Surg Oncol. 22 Suppl 3:S1116–S1124. DOI: 10.1245/s10434-015-4728-4. PMID: 26159442.28. Garcia-Martinez R, Caraceni P, Bernardi M, Gines P, Arroyo V, Jalan R. 2013; Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology. 58:1836–1846. DOI: 10.1002/hep.26338. PMID: 23423799.29. Jeng LB, Chan WL, Teng CF. 2023; Prognostic significance of serum albumin level and albumin-based mono- and combination biomarkers in patients with hepatocellular carcinoma. Cancers (Basel). 15:1005. DOI: 10.3390/cancers15041005. PMID: 36831351. PMCID: PMC9953807.30. Carr B, Guerra V, Karaoğullarından Ü, Akkiz H, Ince V, Isik B, et al. Liver inflammation parameters in relation to survival in patients with hepatocellular carcinoma tumor of differing sizes. J Cancer Sci Ther. 2022; S9:012.31. Lee YH, Hsu CY, Chu CW, Liu PH, Hsia CY, Huang YH, et al. 2014; A new Child-Turcotte-Pugh class 0 for patients with hepatocellular carcinoma: determinants, prognostic impact and ability to improve the current staging systems. PLoS One. 9:e99115. DOI: 10.1371/journal.pone.0099115. PMID: 24906132. PMCID: PMC4048310.32. Kokudo T, Hasegawa K, Amikura K, Uldry E, Shirata C, Yamaguchi T, et al. 2016; Assessment of preoperative liver function in patients with hepatocellular carcinoma - the Albumin-Indocyanine Green Evaluation (ALICE) grade. PLoS One. 11:e0159530. DOI: 10.1371/journal.pone.0159530. PMID: 27434062. PMCID: PMC4951137.33. Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, et al. 1985; Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 56:918–928. DOI: 10.1002/1097-0142(19850815)56:4<918::AID-CNCR2820560437>3.0.CO;2-E. PMID: 2990661.34. Nong X, Zhang Y, Xie J, Liang J, Xie A, Zhang Z. 2023; Evaluation of the up-to-7 criterion for determining the treatment of hepatocellular carcinoma in Barcelona Clinic Liver Cancer stage B: a single-center retrospective cohort study. J Gastrointest Oncol. 14:768–779. DOI: 10.21037/jgo-23-69. PMID: 37201043. PMCID: PMC10186506.35. Shimose S, Kawaguchi T, Iwamoto H, Niizeki T, Shirono T, Tanaka M, et al. 2020; Indication of suitable transarterial chemoembolization and multikinase inhibitors for intermediate stage hepatocellular carcinoma. Oncol Lett. 19:2667–2676. DOI: 10.3892/ol.2020.11399. PMID: 32218817. PMCID: PMC7068224.36. Chan SL, Mo FK, Johnson PJ, Liem GS, Chan TC, Poon MC, et al. 2011; Prospective validation of the Chinese University Prognostic Index and comparison with other staging systems for hepatocellular carcinoma in an Asian population. J Gastroenterol Hepatol. 26:340–347. DOI: 10.1111/j.1440-1746.2010.06329.x. PMID: 21261725.37. Hsu CY, Liu PH, Lee YH, Hsia CY, Huang YH, Lin HC, et al. 2015; Using serum α-fetoprotein for prognostic prediction in patients with hepatocellular carcinoma: what is the most optimal cutoff? PLoS One. 10:e0118825. DOI: 10.1371/journal.pone.0118825. PMID: 25738614. PMCID: PMC4349891.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognostic Significance of Preoperative Controlling Nutritional Status Score in Patients Who Underwent Hepatic Resection for Hepatocellular Carcinoma
- ADV score is a reliable surrogate biomarker of hepatocellular carcinoma in liver resection and transplantation
- Validation of prognostic impact of ADV score for resection of hepatocellular carcinoma: analysis using Korea Liver Cancer Registry Database
- Prospective Validation of the CLIP Score: A New Prognostic System for Patients with Cirrhosis and Hepatocellular Carcinoma
- Preoperative Evaluation and Postoperative Complications with Prognostic Impact of Surgical Resection for the Hepatocellular Carcinoma in Cirrhotics