Korean J Physiol Pharmacol.
2024 Nov;28(6):569-576. 10.4196/kjpp.2024.28.6.569.
Norepinephrine triggers glutamatergic long-term potentiation in hypothalamic paraventricular nucleus magnocellular neuroendocrine cells through postsynaptic ββ1-AR/PKA signaling pathway in vitro in rats
- Affiliations
-
- 1Department of Physiology and Pathophysiology, College of Medicine, Yanbian University, Yanji 133002, China
- 2Institute of Brain Science, Jilin Medical University, Jilin 132013, China
- 3Department of Orthopedics, Affiliated Hospital of Yanbian University, Yanji 133000, China
- 4Department of Physiology, College of Basic Medicine, Jilin Medical University, Jilin 132013, China
- 5Department of Cardiology, Affiliated Hospital of Yanbian University, Yanji 133000, Jilin, China
- KMID: 2560736
- DOI: http://doi.org/10.4196/kjpp.2024.28.6.569
Abstract
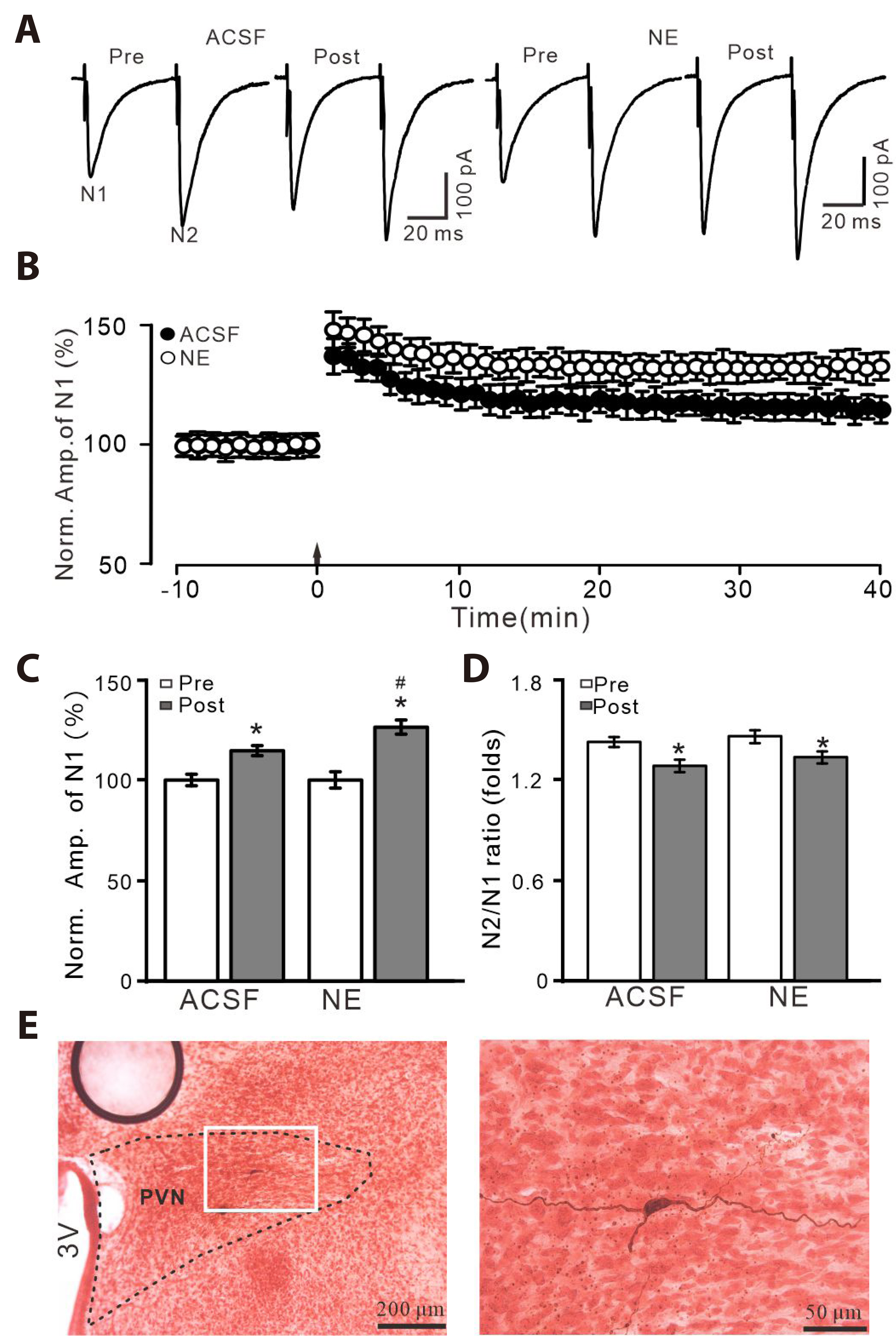
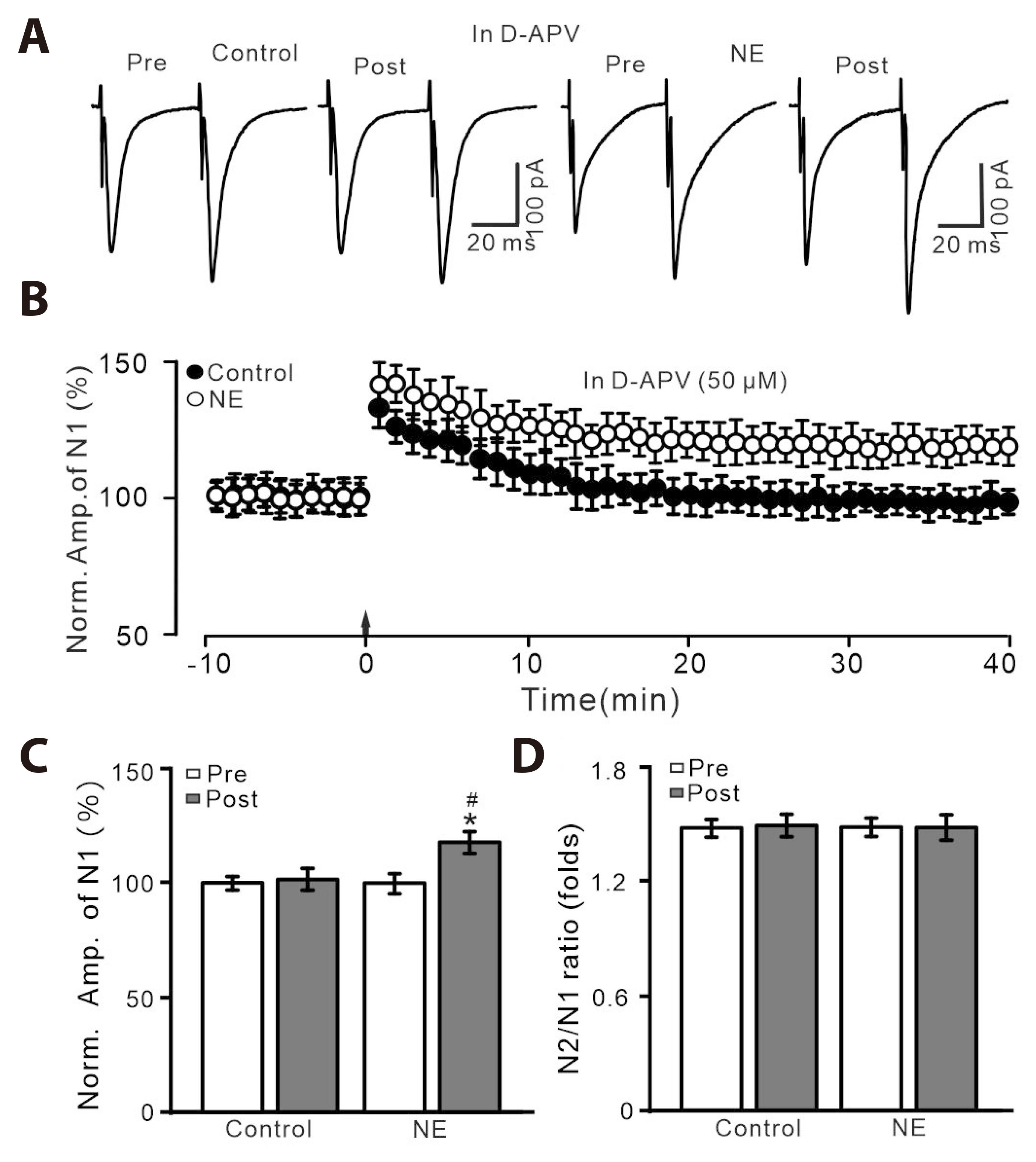
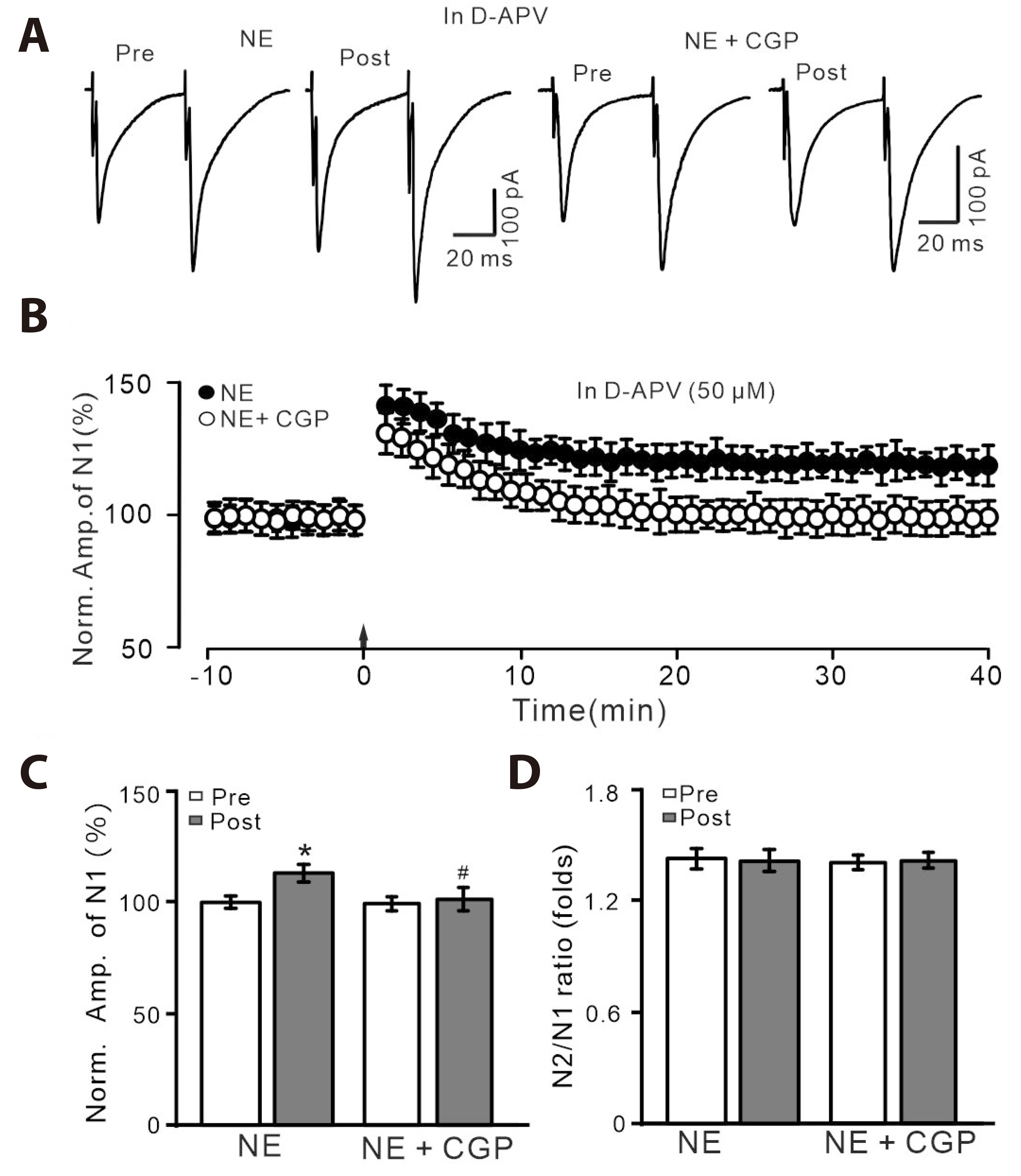
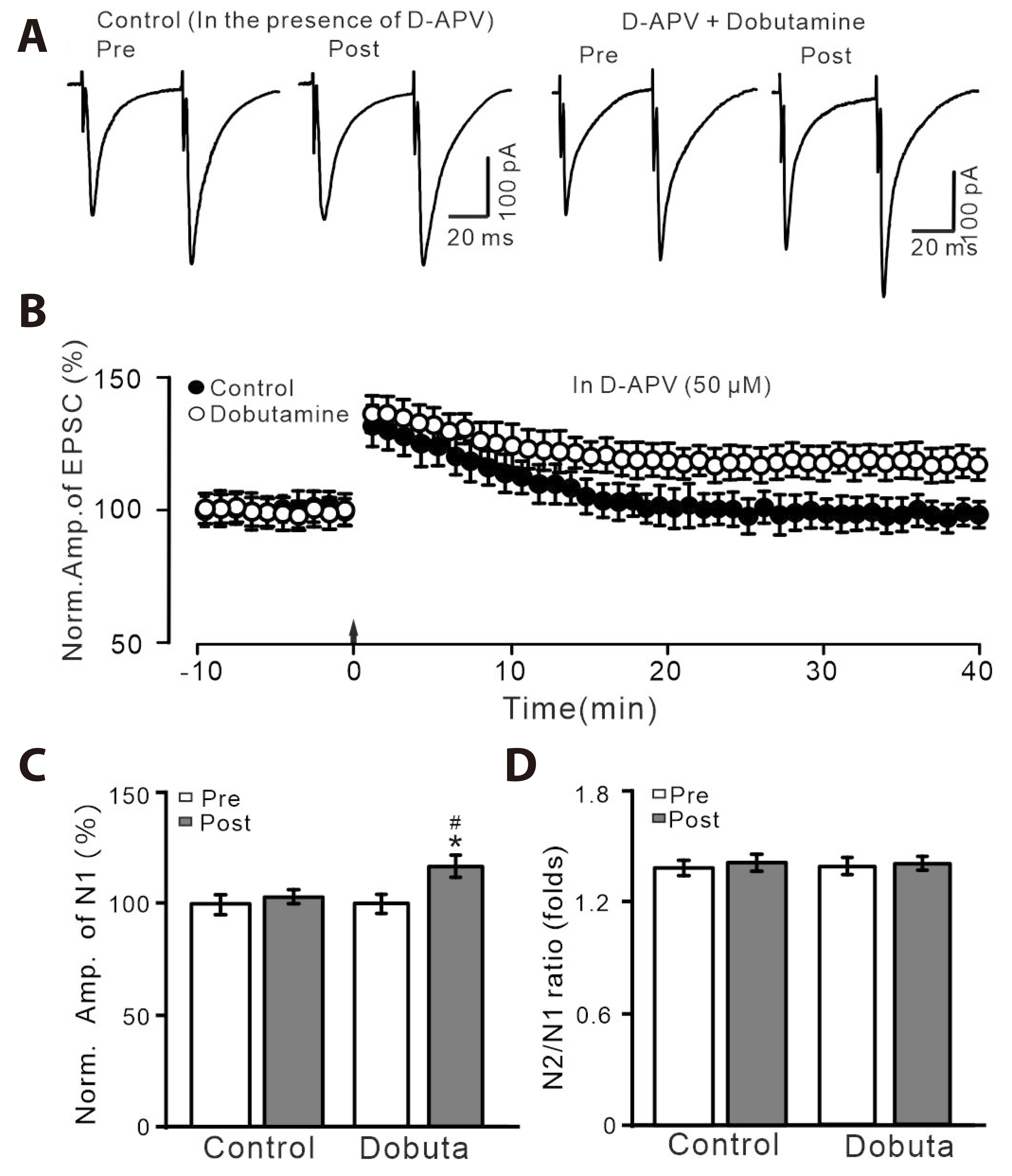
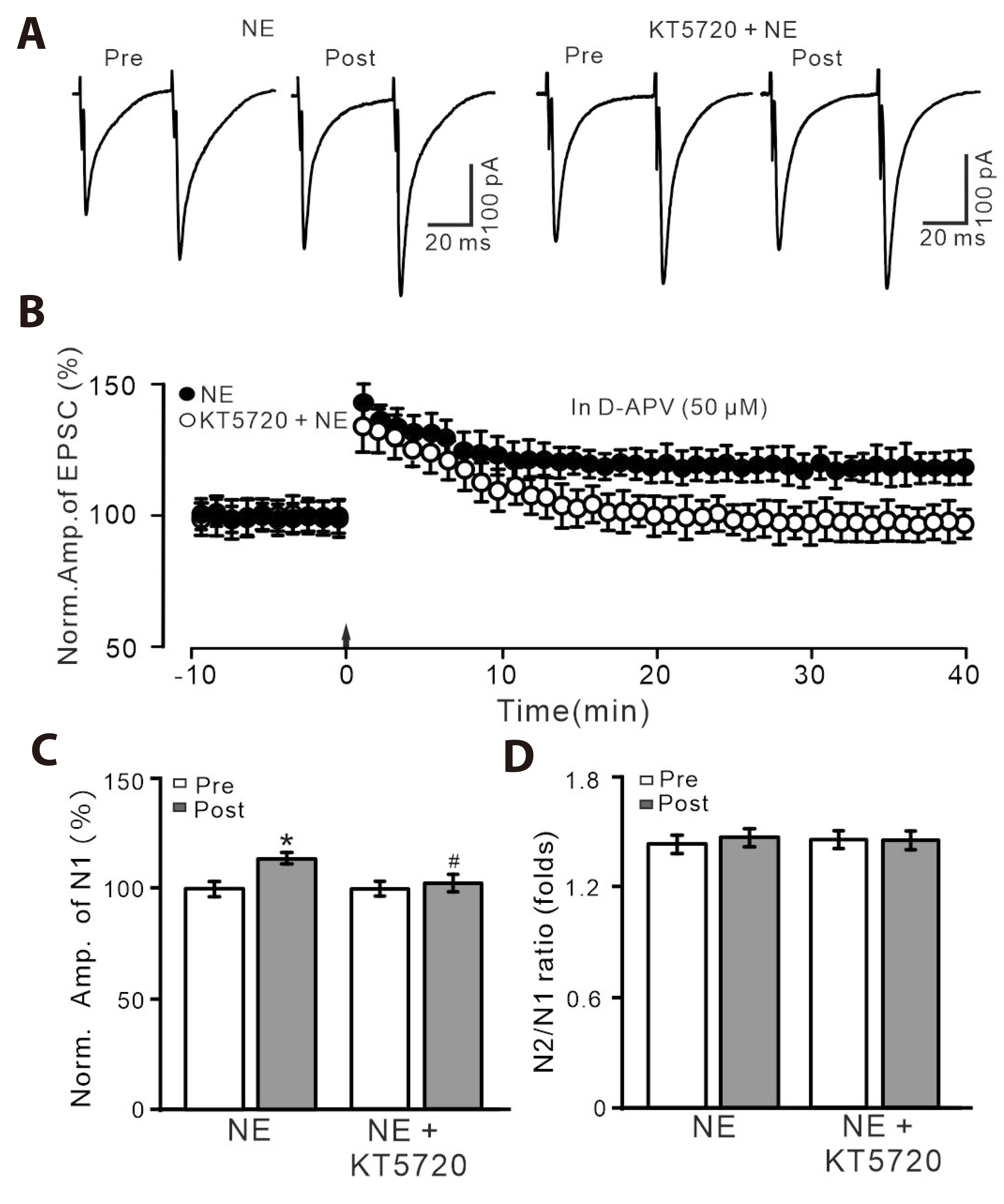
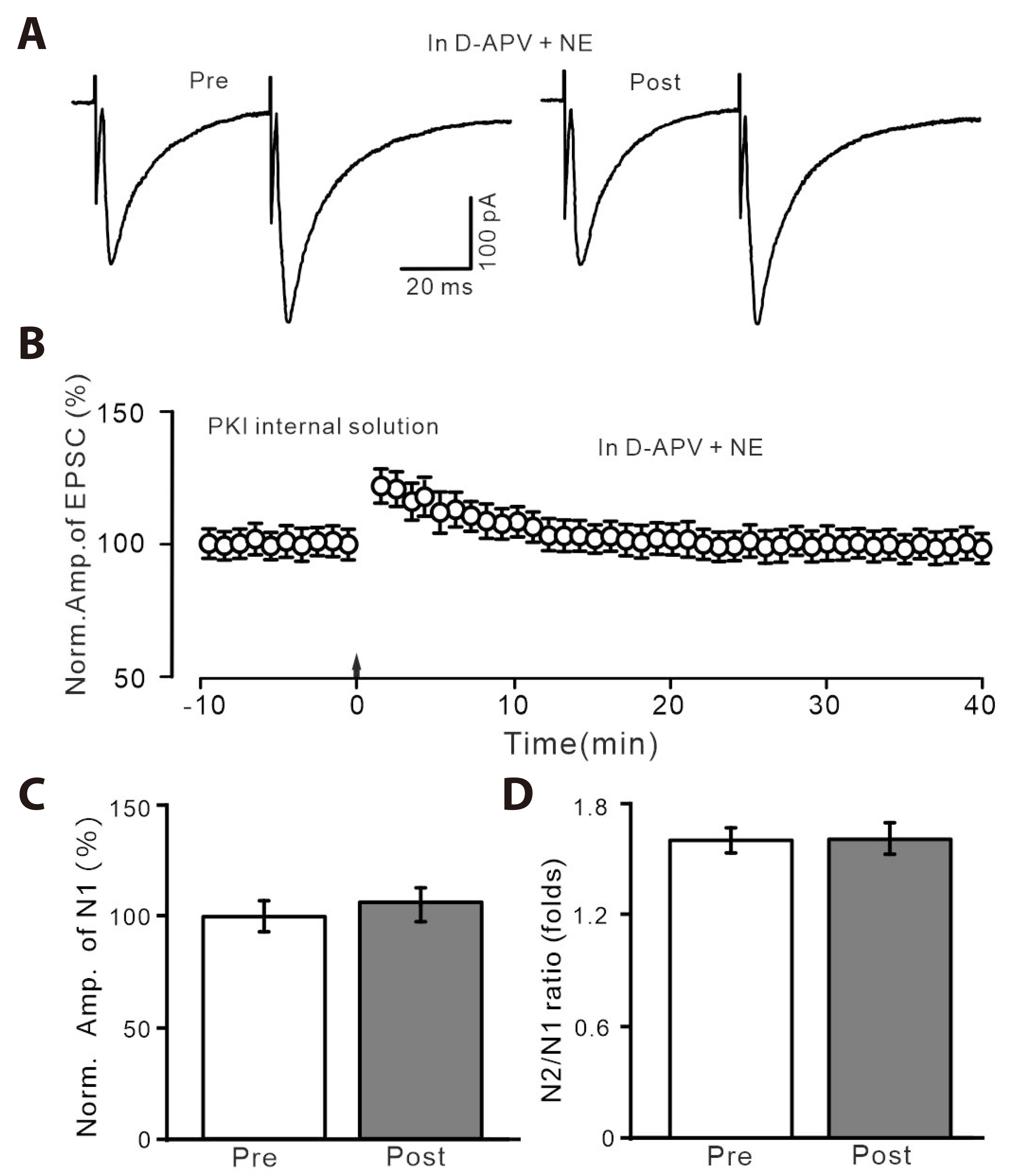
- Norepinephrine (NE) modulates synaptic transmission and long-term plasticity through distinct subtype adrenergic receptor (AR)-mediated-intracellular signaling cascades. However, the role of NE modulates glutamatergic long-term potentiation (LTP) in the hypothalamic paraventricular nucleus (PVN) magnocellular neuroendocrine cells (MNCs) is unclear. We here investigate the effect of NE on high frequency stimulation (HFS)-induced glutamatergic LTP in rat hypothalamic PVN MNCs in vitro, by whole-cell patch-clamp recording, biocytin staining and pharmacological methods. Delivery of HFS induced glutamatergic LTP with a decrease in N2/N1 ratio in the PVN MNCs, which was enhanced by application of NE (100 nM). HFS-induced LTP was abolished by the blockade of N-methyl-D-aspartate receptors (NMDAR) with D-APV, but it was rescued by the application of NE. NE failed to rescue HFS-induced LTP of MNCs in the presence of a selective β1-AR antagonist, CGP 20712. However, application of β1-AR agonist, dobutamine HCl rescued HFS-induced LTP of MNCs in the absence of NMDAR activity. In the absence of NMDAR activity, NE failed to rescue HFS-induced MNC LTP when protein kinase A (PKA) was inhibited by extracellular applying KT5720 or intracellular administration of PKI. These results indicate that NE activates β1-AR and triggers HFS to induce a novel glutamatergic LTP of hypothalamic PVN NMCs via the postsynaptic PKA signaling pathway in vitro in rats.
Keyword
Figure
Reference
-
1. Zhang BB, Jin H, Bing YH, Zhang XY, Chu CP, Li YZ, Qiu DL. 2019; A nitric oxide-dependent presynaptic LTP at glutamatergic synapses of the PVN magnocellular neurosecretory cells in vitro in rats. Front Cell Neurosci. 13:283. DOI: 10.3389/fncel.2019.00283. PMID: 31316353. PMCID: PMC6610542.2. Csáki A, Kocsis K, Halász B, Kiss J. 2000; Localization of glutamatergic/aspartatergic neurons projecting to the hypothalamic paraventricular nucleus studied by retrograde transport of [3H]D-aspartate autoradiography. Neuroscience. 101:637–655. DOI: 10.1016/S0306-4522(00)00411-5. PMID: 11113313.3. van den Pol AN, Wuarin JP, Dudek FE. 1990; Glutamate, the dominant excitatory transmitter in neuroendocrine regulation. Science. 250:1276–1278. DOI: 10.1126/science.1978759. PMID: 1978759.4. Decavel C, Van den Pol AN. 1990; GABA: a dominant neurotransmitter in the hypothalamus. J Comp Neurol. 302:1019–1037. DOI: 10.1002/cne.903020423. PMID: 2081813.5. Wuarin JP, Dudek FE. 1993; Patch-clamp analysis of spontaneous synaptic currents in supraoptic neuroendocrine cells of the rat hypothalamus. J Neurosci. 13:2323–2331. DOI: 10.1523/JNEUROSCI.13-06-02323.1993. PMID: 8099123. PMCID: PMC6576514.6. Stern JE, Galarreta M, Foehring RC, Hestrin S, Armstrong WE. 1999; Differences in the properties of ionotropic glutamate synaptic currents in oxytocin and vasopressin neuroendocrine neurons. J Neurosci. 19:3367–3375. DOI: 10.1523/JNEUROSCI.19-09-03367.1999. PMID: 10212296. PMCID: PMC6782249.7. Panatier A, Gentles SJ, Bourque CW, Oliet SH. 2006; Activity-dependent synaptic plasticity in the supraoptic nucleus of the rat hypothalamus. J Physiol. 573:711–721. DOI: 10.1113/jphysiol.2006.109447. PMID: 16613872. PMCID: PMC1779752.8. Salter EW, Sunstrum JK, Matovic S, Inoue W. 2018; Chronic stress dampens excitatory synaptic gain in the paraventricular nucleus of the hypothalamus. J Physiol. 596:4157–4172. DOI: 10.1113/JP275669. PMID: 29901836. PMCID: PMC6117590.9. Matsuura T, Kawasaki M, Suzuki H, Fujitani T, Baba K, Nishimura H, Ikeda N, Yamanaka Y, Tsukamoto M, Yoshimi Y, Ohnishi H, Ueta Y, Sakai A. 2023; Nitric oxide synthase contributes to the maintenance of LTP in the oxytocin-mRFP1 neuron of the rat hypothalamus. J Neuroendocrinol. 35:e13340. DOI: 10.1111/jne.13340. PMID: 37776071.10. Maguire J. 2014; Stress-induced plasticity of GABAergic inhibition. Front Cell Neurosci. 8:157. DOI: 10.3389/fncel.2014.00157. PMID: 24936173. PMCID: PMC4047962.11. Stanton LM, Price AJ, Manning EE. 2023; Hypothalamic corticotrophin releasing hormone neurons in stress-induced psychopathology: revaluation of synaptic contributions. J Neuroendocrinol. 35:e13268. DOI: 10.1111/jne.13268. PMID: 37078436.12. Herman JP, Tasker JG. 2016; Paraventricular hypothalamic mechanisms of chronic stress adaptation. Front Endocrinol (Lausanne). 7:137. DOI: 10.3389/fendo.2016.00137. PMID: 27843437. PMCID: PMC5086584.13. Chappell PB, Smith MA, Kilts CD, Bissette G, Ritchie J, Anderson C, Nemeroff CB. 1986; Alterations in corticotropin-releasing factor-like immunoreactivity in discrete rat brain regions after acute and chronic stress. J Neurosci. 6:2908–2914. DOI: 10.1523/JNEUROSCI.06-10-02908.1986. PMID: 3020187. PMCID: PMC6568795.14. Ma XM, Lightman SL, Aguilera G. 1999; Vasopressin and corticotropin-releasing hormone gene responses to novel stress in rats adapted to repeated restraint. Endocrinology. 140:3623–3632. DOI: 10.1210/endo.140.8.6943. PMID: 10433220.15. Bartsch JC, von Cramon M, Gruber D, Heinemann U, Behr J. 2021; Stress-induced enhanced long-term potentiation and reduced threshold for N-methyl-D-aspartate receptor- and β-adrenergic receptor-mediated synaptic plasticity in rodent ventral subiculum. Front Mol Neurosci. 14:658465. DOI: 10.3389/fnmol.2021.658465. PMID: 33967694. PMCID: PMC8100191.16. Chay A, Zamparo I, Koschinski A, Zaccolo M, Blackwell KT. 2016; Control of βAR- and N-methyl-D-aspartate (NMDA) receptor-dependent cAMP dynamics in hippocampal neurons. PLoS Comput Biol. 12:e1004735. DOI: 10.1371/journal.pcbi.1004735. PMID: 26901880. PMCID: PMC4763502.17. Han SK, Chong W, Li LH, Lee IS, Murase K, Ryu PD. 2002; Noradrenaline excites and inhibits GABAergic transmission in parvocellular neurons of rat hypothalamic paraventricular nucleus. J Neurophysiol. 87:2287–2296. DOI: 10.1152/jn.2002.87.5.2287. PMID: 11976368.18. Inoshita T, Hirano T. 2021; Norepinephrine facilitates induction of long-term depression through β-adrenergic receptor at parallel fiber-to-Purkinje cell synapses in the flocculus. Neuroscience. 462:141–150. DOI: 10.1016/j.neuroscience.2020.05.037. PMID: 32502572.19. Cui LN, Sun N, Li BX, Wang LF, Zhang XY, Qiu DL, Chu CP. 2020; Noradrenaline inhibits complex spikes activity via the presynaptic PKA signaling pathway in mouse cerebellar slices. Neurosci Lett. 729:135008. DOI: 10.1016/j.neulet.2020.135008. PMID: 32344107.20. Wang JY, Weng WC, Wang TQ, Liu Y, Qiu DL, Wu MC, Chu CP. 2023; Noradrenaline depresses facial stimulation-evoked cerebellar MLI-PC synaptic transmission via α2-AR/PKA signaling cascade in vivo in mice. Sci Rep. 13:15908. DOI: 10.1038/s41598-023-42975-5. PMID: 37741947. PMCID: PMC10517918.21. Yamaguchi N, Mimura K, Okada S. 2019; GABAB receptors in the hypothalamic paraventricular nucleus mediate β-adrenoceptor-induced elevations of plasma noradrenaline in rats. Eur J Pharmacol. 848:88–95. DOI: 10.1016/j.ejphar.2019.01.029. PMID: 30685430.22. Domingos-Souza G, Martinez D, Sinkler S, Heesch CM, Kline DD. 2021; Alpha adrenergic receptor signaling in the hypothalamic paraventricular nucleus is diminished by the chronic intermittent hypoxia model of sleep apnea. Exp Neurol. 335:113517. DOI: 10.1016/j.expneurol.2020.113517. PMID: 33132201. PMCID: PMC7750300.23. Chu CP, Jin WZ, Bing YH, Jin QH, Kannan H, Qiu DL. 2013; Effects of stresscopin on rat hypothalamic paraventricular nucleus neurons in vitro. PLoS One. 8:e53863. DOI: 10.1371/journal.pone.0053863. PMID: 23349753. PMCID: PMC3548845.24. Tasker JG, Dudek FE. 1991; Electrophysiological properties of neurones in the region of the paraventricular nucleus in slices of rat hypothalamus. J Physiol. 434:271–293. DOI: 10.1113/jphysiol.1991.sp018469. PMID: 2023120. PMCID: PMC1181417.25. O'Dell TJ, Connor SA, Guglietta R, Nguyen PV. 2015; β-Adrenergic receptor signaling and modulation of long-term potentiation in the mammalian hippocampus. Learn Mem. 22:461–471. DOI: 10.1101/lm.031088.113. PMID: 26286656. PMCID: PMC4561407.26. Laing M, Bashir ZI. 2014; β-Adrenoceptors and synaptic plasticity in the perirhinal cortex. Neuroscience. 273:163–173. DOI: 10.1016/j.neuroscience.2014.04.070. PMID: 24836853. PMCID: PMC4067743.27. O'Dell TJ, Connor SA, Gelinas JN, Nguyen PV. 2010; Viagra for your synapses: enhancement of hippocampal long-term potentiation by activation of beta-adrenergic receptors. Cell Signal. 22:728–736. DOI: 10.1016/j.cellsig.2009.12.004. PMID: 20043991. PMCID: PMC2826554.28. Wang M, Ramasamy VS, Kang HK, Jo J. 2020; Oleuropein promotes hippocampal LTP via intracellular calcium mobilization and Ca2+-permeable AMPA receptor surface recruitment. Neuropharmacology. 176:108196. DOI: 10.1016/j.neuropharm.2020.108196. PMID: 32598912.29. Park P, Georgiou J, Sanderson TM, Ko KH, Kang H, Kim JI, Bradley CA, Bortolotto ZA, Zhuo M, Kaang BK, Collingridge GL. 2021; PKA drives an increase in AMPA receptor unitary conductance during LTP in the hippocampus. Nat Commun. 12:413. DOI: 10.1038/s41467-020-20523-3. PMID: 33462202. PMCID: PMC7814032.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical Localization of Brain Natriuretic Peptide in the Hypothalamus of the Rat
- Fasting-induced Down-regulation of NADPH-diaphorase in the Magnocellular PVN of Rats
- Postnatal Development of Brain Natriuretic Peptide-immunoreactive Neuron in the Hypothalamus of the Rat
- Effect of Chronic Alcohol Intake on Vasopressin and Oxytocin-containing Neurons in the Paraventricular and Supraoptic Nucleus of the Rat Hypothalamus
- Study on CNS Oxytocinergic Pathway Projecting to the Mammary Nerve of the Rat