J Korean Med Sci.
2024 Oct;39(40):e311. 10.3346/jkms.2024.39.e311.
Healthcare Experts’ Advisory Unit and Support (HAUS) Program for Medical Device Development in Korea: Introduction of Clinical Unmet Needs-Based Intended Use Establishment (CLUE) Templates
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Soonchunhyang University College of Medicine, Bucheon, Korea
- 2Research Center, Korean Academy of Medical Sciences (KAMS), Seoul, Korea
- 3Medical Device Clinical Research Center, Soonchunhyang University, Bucheon, Korea
- 4Convergence Division, Korea Medical Device Development Fund (KMDF), Seoul, Korea
- 5Department of Rehabilitation Medicine, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, Korea
- 6Department of Family Medicine, Kangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea
- 7Department of Medical Engineering, Dongguk University College of Medicine, Goyang, Korea
- 8Department of Biomedical Engineering, University of Ulsan College of Medicine, Seoul, Korea
- 9Department of Otorhinolaryngology-Head and Neck Surgery, Medical Device Usability Test Center, Guro Hospital, Korea University College of Medicine, Seoul, Korea
- 10Department of Orthopaedic Surgery, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2560665
- DOI: http://doi.org/10.3346/jkms.2024.39.e311
Abstract
- Background
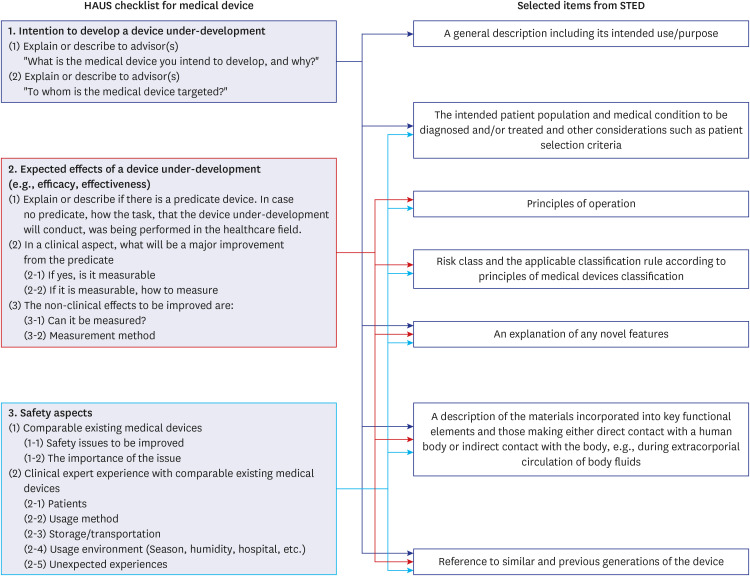
A clear and precise definition of the “intended use” in developing new medical devices can determine the success of entering the healthcare market. For this, practical collaboration between the clinical and engineering experts is necessary, and an appropriate tool is required for effective information collection and decision-making in the process.
Methods
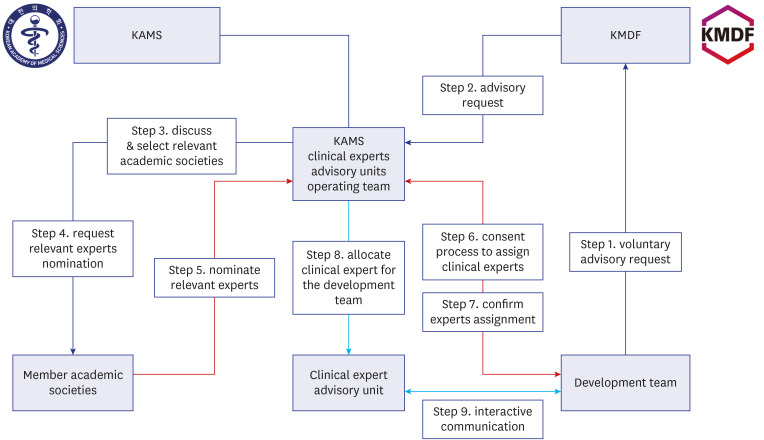
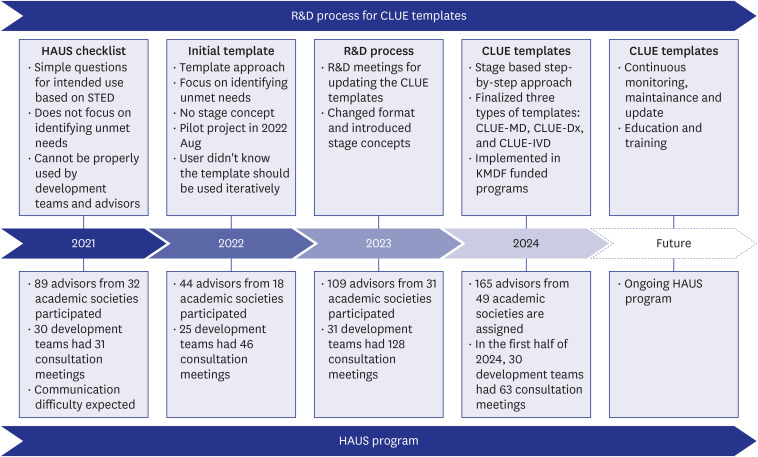
The Korean Academy of Medical Sciences, in cooperation with the Korean Medical Device Development Fund, implemented the Healthcare Experts’ Advisory Unit and Support (HAUS) program to match advisory clinical experts in medical device development projects. Three and five collaborative academic conferences were held in 2022 and 2023 to raise awareness of the HAUS program. In the consultation meeting, checklists were used to facilitate communications and satisfaction surveys were conducted afterward. Then, the results of the consultation meetings were compiled to build an integrated document.
Results
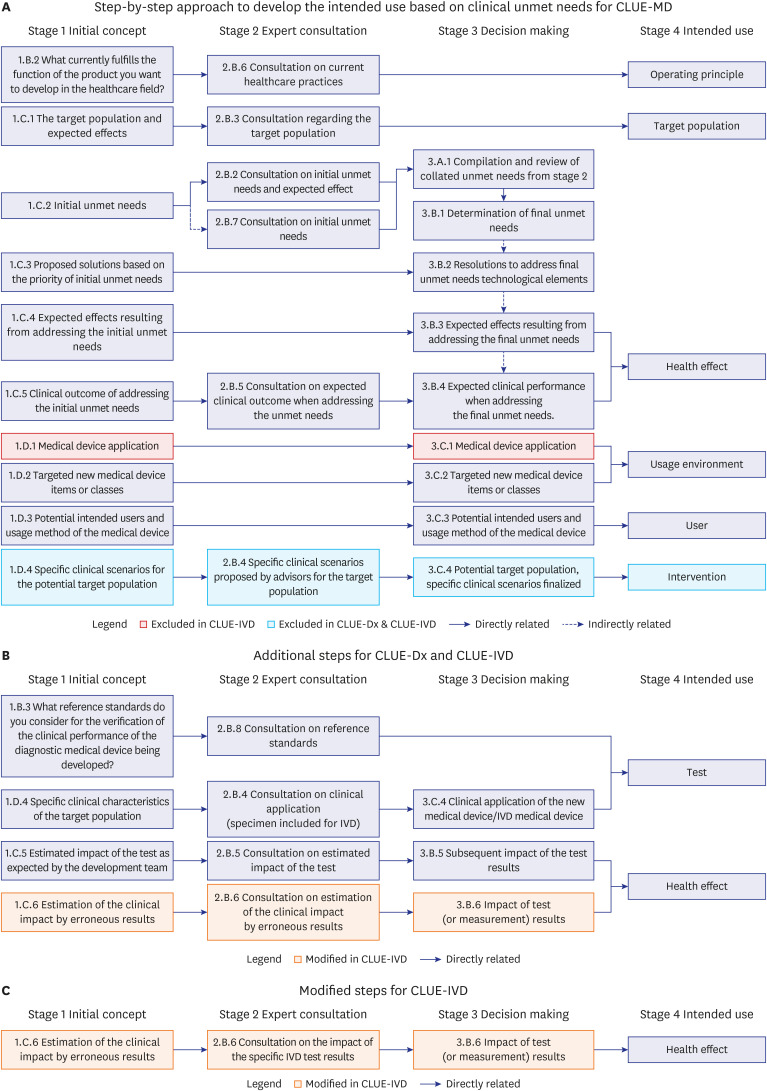
The HAUS program was conducted with a gradually increasing number of consultation sessions from 31 in 2021 to 128 in 2023. The medical device development teams (development teams) expressed a higher level of satisfaction (91.4% to 100%) compared to the advisors (clinical experts) (78.6% to 100%) across the survey items. Based on the experiences and observations of the HAUS consultation meetings, the “Clinical Unmet Needs-based Intended Use Establishment (CLUE) templates” were developed, which were purposes to improve communication efficiency and to support a systematic approach in establishing the intended use. The CLUE process comprises four main stages for processing: Stage 1, Initial Concept; Stage 2, Expert Consultation; Stage 3, Decision-making; and Stage 4, Intended Use.
Conclusion
The HAUS program seemed to be helpful for the development teams by providing opinions of clinical experts. And the resultant product, the CLUE templates have been proposed to facilitate collaboration between the development teams and the advisors and to define robust clinical intended use.
Keyword
Figure
Reference
-
1. National Research Council (US) Committee on the Role of Human Factors in Home Health Care. The role of human factors in home health care: workshop summary. Updated 2010. Accessed July 26, 2024. https://www.ncbi.nlm.nih.gov/books/NBK210047/ .2. Aronson JK, Heneghan C, Ferner RE. Medical devices: definition, classification, and regulatory implications. Drug Saf. 2020; 43(2):83–93. PMID: 31845212.3. Chandan B, Balamuralidhara V, Gowrav M, Motupalli V. Applications of medical devices in healthcare industry. J Evol Med Dent Sci. 2021; 10(38):3419–3424.4. Global Harmonization Task Force (GHTF). GHTF/SG1/N071:2012: definition of the terms 'Medical Device' and 'In Vitro Diagnostic (IVD) Medical Device'. Updated 2012. Accessed September 26, 2024. https://www.imdrf.org/sites/default/files/docs/ghtf/final/sg1/technical-docs/ghtf-sg1-n071-2012-definition-of-terms-120516.pdf .5. Warty RR, Smith V, Patabendige M, Fox D, Mol B. Clarifying the unmet clinical need during medical device innovation in women’s health - a narrative review. Health Care Women Int. 2024; 45(7):811–839. PMID: 37000043.6. Avari Silva JN. Clinical needs should drive innovation. J Cardiovasc Electrophysiol. 2023; 34(7):1587–1588. PMID: 37313795.7. Goldberg J. Identifying problems and unmet clinical needs: who to talk to and where to look. IEEE Pulse. 2023; 14(1):28–30.8. McCarthy AD, Sproson L, Wells O, Tindale W. Unmet needs: relevance to medical technology innovation? J Med Eng Technol. 2015; 39(7):382–387.9. Food and Drug Administration (US). Design control guidance for medical device manufacturers. Updated 1997. Accessed July 24, 2024. https://www.fda.gov/media/116573/download .10. Yock PG, Zenios S, Makower J, Briton TJ, Kumar UN, Watkins FTJ. Biodesign. Cambridge, UK: Cambridge University Press;2015.11. D’Souza R, Tauheed A, Jangir R, Chachra P, Sirdesai R. A structured process to identify unmet needs for medical device innovation in obstetrics and gynaecology. J Biomed Res Prac. 2018; 2(1):100009.12. Yang MY, Gemba K, Tamada S. Training innovators at the Stanford Biodesign program and its implications. In : Proceedings of the 2016 Portland International Conference on Management of Engineering and Technology (PICMET); 2016 September 4–8; New York, NY, USA: Institute of Electrical and Electronics Engineers;2016.13. Weigl BH, Gaydos CA, Kost G, Beyette FR Jr, Sabourin S, Rompalo A, et al. The value of clinical needs assessments for point-of-care diagnostics. Point Care. 2012; 11(2):108–113. PMID: 23935405.14. International Medical Device Regulators Forum (IMDRF). IMDRF/GRRP WG/N47 FINAL:2024 (edition 2): essential principles of safety and performance of medical devices and IVD medical devices. Updated 2024. Accessed April 1, 2024. https://www.imdrf.org/documents/essential-principles-safety-and-performance-medical-devices-and-ivd-medical-devices .15. International Organization for Standardization (ISO). ISO 14971:2019: medical devices—application of risk management to medical devices. Updated 2019. Accessed July 29, 2024. https://www.iso.org/standard/72704.html .16. Lee JW. About Korean Academy of Medical Sciences. Updated 2021. Accessed March 20, 2024. https://www.kams.or.kr/eng/about/sub1.php .17. Korea Medical Device Development Fund. Business overview. Accessed April 1, 2024. https://kmdf.org/summary .18. Global Harmonization Task Force (GHTF). GHTF/SG1/N011:2008: summary technical documentation for demonstrating conformity to the essential principles of safety and performance of medical devices (STED). Updated 2008. Accessed July 29, 2024. https://www.imdrf.org/sites/default/files/docs/ghtf/archived/SG1/technical-docs/ghtf-sg1-n011-2008-principles-safety-performance-medical-devices-080221.pdf .19. International Medical Device Regulators Forum (IMDRF). IMDRF MDCE WG/N57FINAL:2019: clinical investigation. Updated 2019. Accessed April 1, 2024. https://www.imdrf.org/documents/clinical-investigation .20. National Evidence-based Healthcare Collaborating Agency (NECA). New health technology assessment system of South Korea. Accessed July 26, 2024. https://nhta.neca.re.kr/nhta/eng/nhtaENG0101VA.ecg .21. Richardson WS, Wilson MC, Nishikawa J, Hayward RS. The well-built clinical question: a key to evidence-based decisions. ACP J Club. 1995; 123(3):A12–A13.22. International Organization for Standardization (ISO). ISO 13485:2016: medical devices—quality management systems—requirements for regulatory purposes. Updated 2016. Accessed July 29, 2024. https://www.iso.org/standard/59752.html .23. Dixon J. The “market pull” versus “technology push” continuum of engineering education. In : Proceedings of the 2001 American Society for Engineering Education Annual Conference & Exposition; Washington, D.C., USA: American Society for Engineering Education;2001.24. Steinberger JD, Denend L, Azagury DE, Brinton TJ, Makower J, Yock PG. Needs-based innovation in interventional radiology: the Biodesign process. Tech Vasc Interv Radiol. 2017; 20(2):84–89. PMID: 28673651.25. Schwartz JG, Kumar UN, Azagury DE, Brinton TJ, Yock PG. Needs-based innovation in cardiovascular medicine: the Stanford Biodesign process. JACC Basic Transl Sci. 2016; 1(6):541–547. PMID: 30167537.26. International Electrotechnical Commission (IEC). IEC 62366-1:2015: medical devices—part 1: application of usability engineering to medical devices. Updated 2015. Accessed July 29, 2024. https://www.iso.org/standard/63179.html .27. International Electrotechnical Commission (IEC). IEC 62304:2006: medical device software—software life cycle processes. Updated 2006. Accessed July 29, 2024. https://www.iso.org/standard/38421.html .28. Chaturvedi J, Logan A, Narayan G, Kuttappa S. A structured process for unmet clinical need analysis for medical device innovation in India: early experiences. BMJ Innovations. 2015; 1(3):81–87.29. Markiewicz K. Health Technology Assessment of Medical Devices During Development [Dissertation]. Enschede, The Netherlands: University of Twente;2017.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development and Evaluation of a Web-based Support Program for the Maternal Role of Primiparas
- Analysis of pregnant and breast-feeding women’s unmet healthcare needs
- Implementation of Device Independent ECG Reporting System Using Web Development Framework
- Factors Affecting Unmet Healthcare Needs among Adults with Chronic Diseases
- Are medical students being properly cared for? A question for the current student advisory program