J Korean Med Sci.
2024 Nov;39(42):e274. 10.3346/jkms.2024.39.e274.
Reported Adverse Events and Associated Factors in Korean Coronavirus Disease 2019 Vaccinations
- Affiliations
-
- 1COVID-19 Vaccine Safety Research Center, Seoul, Korea
- 2Department of Preventive Medicine, College of Medicine, Graduate Program in System Health Science & Engineering, Ewha Womans University, Seoul, Korea
- 3Department of Health Convergence, College of Science and Industry Convergence, Ewha Womans University, Seoul, Korea
- 4Clinical Trial Center, Ewha Womans University Mokdong Hospital, Seoul, Korea
- 5National Academy of Medicine of Korea, Seoul, Korea
- 6Graduate School of Industrial Pharmaceutical Science of Pharmacy, Ewha Womans University, Seoul, Korea
- 7Department of Preventive Medicine, College of Medicine, Chung-Ang University, Seoul, Korea
- KMID: 2560623
- DOI: http://doi.org/10.3346/jkms.2024.39.e274
Abstract
- Background
Despite their effectiveness, coronavirus disease 2019 (COVID-19) vaccines have been associated with adverse effects, underscoring the importance of continuous surveillance to ensure vaccine safety and effective management of public health. Herein, the characteristics and risk factors of vaccine-related adverse events (AEs) were identified to gain an in-depth understanding of vaccine safety by investigating the impact of the vaccination dose on changes in post-vaccination AEs.
Methods
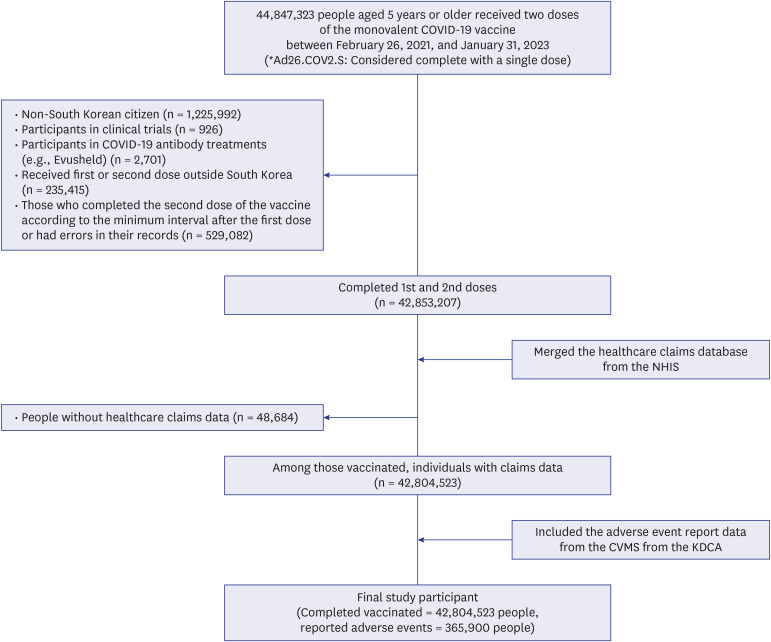
Herein, a linked database of COVID-19 vaccination records from the Korea Disease Control and Prevention Agency, AE reports from the COVID-19 Vaccination Management System, and healthcare claims from the National Health Insurance Service, targeting ≥ 5-year-old individuals, was utilized (study duration = February 26, 2021, to January 31, 2023). The frequency and severity of reported post-vaccination AEs were evaluated. Furthermore, we specifically explored AEs in relation to the cumulative dosage of vaccines administered while evaluating associated risk factors.
Results
During the observation period, 42,804,523 individuals completed the COVID-19 vaccination series, with 365,900 reporting AEs, with headache, muscle pain, and fever being the most frequently reported. Notably, the AE reports were approximately twice as high for women than for men, which was further exacerbated following both doses. Analysis by age group revealed that AE reports were lower among children, adolescents, and older adults than in the middleaged cohort (age = 50–64 years), with higher reports observed for 18–49-year-old individuals. Additionally, a higher risk of reporting was identified among individuals with lower socioeconomic status compared with those of middle socioeconomic status. Excluding dementia, the risk of reporting AEs was high in individuals with underlying diseases compared with those without, for instance, the risk of reporting AEs following two-dose vaccinations was approximately twice as high in individuals with chronic obstructive pulmonary disease and asthma.
Conclusion
These findings indicate that women, younger people, those with a lower socioeconomic status, and those with underlying health conditions reported a higher incidence of AEs following COVID-19 vaccinations. This emphasizes the need for continued monitoring to ensure safe vaccination and address vaccine-related anxiety and fear, especially within the aforementioned groups.
Keyword
Figure
Reference
-
1. Sohrabi C, Alsafi Z, O’Neill N, Khan M, Kerwan A, Al-Jabir A, et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19). Int J Surg. 2020; 76:71–76. PMID: 32112977.2. Choi K, Sim S, Choi J, Park C, Uhm Y, Lim E, et al. Changes in handwashing and hygiene product usage patterns in Korea before and after the outbreak of COVID-19. Environ Sci Eur. 2021; 33(1):79. PMID: 34249592.3. Odusanya OO, Odugbemi BA, Odugbemi TO, Ajisegiri WS. COVID-19: a review of the effectiveness of non-pharmacological interventions. Niger Postgrad Med J. 2020; 27(4):261–267. PMID: 33154276.4. World Health Organization. WHO issues its first emergency use validation for a COVID-19 vaccine and emphasizes need for equitable global access. Updated 2020. Accessed January 26, 2024. https://www.who.int/news/item/31-12-2020-who-issues-its-first-emergency-use-validation-for-a-covid-19-vaccine-and-emphasizes-need-for-equitable-global-access .5. Mathieu E, Ritchie H, Rodés-Guirao L, Appel C, Giattino C, Hasell J, et al. Coronavirus pandemic (COVID-19). Updated 2020. Accessed January 11, 2024. https://ourworldindata.org/coronavirus .6. Korea Disease Control and Prevention Agency. Press release: COVID-19 vaccination to begin this week. Updated 2021. Accessed May 15, 2024. https://www.kdca.go.kr/board/board.es?mid=a30402000000&bid=0030 .7. CoronaBoard (KR). COVID-19 real-time situation board. Updated 2023. Accessed January 11, 2024. https://coronaboard.kr/ .8. Aouissi HA, Kechebar MS, Ababsa M, Roufayel R, Neji B, Petrisor AI, et al. The importance of behavioral and native factors on COVID-19 infection and severity: insights from a preliminary cross-sectional study. Healthcare (Basel). 2022; 10(7):1341. PMID: 35885867.9. Anderson RM, May RM. Vaccination and herd immunity to infectious diseases. Nature. 1985; 318(6044):323–329. PMID: 3906406.10. Tsang RS, Joy M, Byford R, Robertson C, Anand SN, Hinton W, et al. Adverse events following first and second dose COVID-19 vaccination in England, October 2020 to September 2021: a national vaccine surveillance platform self-controlled case series study. Euro Surveill. 2023; 28(3):2200195. PMID: 36695484.11. Mallhi TH, Khan YH, Butt MH, Salman M, Tanveer N, Alotaibi NH, et al. Surveillance of side effects after two doses of COVID-19 vaccines among patients with comorbid conditions: a sub-cohort analysis from Saudi Arabia. Medicina (Kaunas). 2022; 58(12):1799. PMID: 36557002.12. Kaswandani N, Medise BE, Leonard E, Satari HI, Sundoro J, Hadinegoro SR, et al. Safety profile of inactivated COVID-19 in healthy adults aged ≥ 18 years: a passive surveillance in Indonesia. PLoS One. 2023; 18(10):e0286484. PMID: 37824453.13. Zhou W, Pool V, Iskander JK, English-Bullard R, Ball R, Wise RP, et al. Surveillance for safety after immunization: Vaccine Adverse Event Reporting System (VAERS)--United States, 1991-2001. MMWR Surveill Summ. 2003; 52(1):1–24.14. Gee J, Marquez P, Su J, Calvert GM, Liu R, Myers T, et al. First month of COVID-19 vaccine safety monitoring — United States, December 14, 2020–January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021; 70(8):283–288. PMID: 33630816.15. Wolff K. COVID-19 vaccination intentions: the theory of planned behavior, optimistic bias, and anticipated regret. Front Psychol. 2021; 12:648289. PMID: 34220620.16. Kim S, Ko M, Heo Y, Lee YK, Kwon Y, Choi SK, et al. Safety surveillance of the NVX-CoV2373 COVID-19 vaccine among Koreans aged 18 years and over. Vaccine. 2023; 41(35):5066–5071. PMID: 37422379.17. Seong SC, Kim YY, Khang YH, Park JH, Kang HJ, Lee H, et al. Data resource profile: the National Health Information Database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017; 46(3):799–800. PMID: 27794523.18. Korea Disease Control and Prevention Agency. Guideline for adverse events following COVID-19 immunization, 2-2nd edition. Updated 2022. Accessed December 11, 2023. https://www.kdca.go.kr/filepath/boardSyview.es?bid=0019&list_no=720158&seq=1 .19. Green MS, Peer V, Magid A, Hagani N, Anis E, Nitzan D. HaGani N, Anis E, Nitzan D. Gender differences in adverse events following the Pfizer-BioNTech COVID-19 vaccine. Vaccines (Basel). 2022; 10(2):233. PMID: 35214694.20. Xiong X, Yuan J, Li M, Jiang B, Lu ZK. Age and gender disparities in adverse events following COVID-19 vaccination: real-world evidence based on big data for risk management. Front Med (Lausanne). 2021; 8:700014. PMID: 34350199.21. Barry V, Dasgupta S, Weller DL, Kriss JL, Cadwell BL, Rose C, et al. Patterns in COVID-19 vaccination coverage, by social vulnerability and Urbanicity — United States, December 14, 2020–May 1, 2021. MMWR Morb Mortal Wkly Rep. 2021; 70(22):818–824. PMID: 34081685.22. Bahat KA. Overview of COVID-19 vaccine and investigation of side effects in patients over 65 years of age with chronic kidney disease. Eur J Geriatr Gerontol. 2022; 4(2):91–96.23. World Health Organization. Statement on the fifteenth meeting of the IHR (2005) Emergency Committee on the COVID-19 pandemic. Updated 2023. Accessed May 15, 2024. https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(COVID-19)-pandemic .24. Marra AR, Kobayashi T, Callado GY, Pardo I, Gutfreund MC, Hsieh MK, et al. The effectiveness of COVID-19 vaccine in the prevention of post-COVID conditions: a systematic literature review and meta-analysis of the latest research. Antimicrob Steward Healthc Epidemiol. 2023; 3(1):e168. PMID: 38028898.25. Pfizer. Pfizer and BioNTech receive U.S. FDA approval for 2023–2024 COVID-19 vaccine. Updated 2023. Accessed May 15, 2024. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-receive-us-fda-approval-2023-2024-covid .26. Link-Gelles R, Ciesla AA, Mak J, Miller JD, Silk BJ, Lambrou AS, et al. Early estimates of updated 2023-2024 (Monovalent XBB.1.5) COVID-19 vaccine effectiveness against symptomatic SARS-CoV-2 infection attributable to co-circulating omicron variants among immunocompetent adults - increasing community access to testing program, United States, September 2023-January 2024. MMWR Morb Mortal Wkly Rep. 2024; 73(4):77–83. PMID: 38300853.27. Sherman SM, Smith LE, Sim J, Amlôt R, Cutts M, Dasch H, et al. COVID-19 vaccination intention in the UK: results from the COVID-19 vaccination acceptability study (CoVAccS), a nationally representative cross-sectional survey. Hum Vaccin Immunother. 2021; 17(6):1612–1621. PMID: 33242386.28. Breslin G, Dempster M, Berry E, Cavanagh M, Armstrong NC. COVID-19 vaccine uptake and hesitancy survey in Northern Ireland and Republic of Ireland: applying the theory of planned behaviour. PLoS One. 2021; 16(11):e0259381. PMID: 34788330.29. Solís Arce JS, Warren SS, Meriggi NF, Scacco A, McMurry N, Voors M, et al. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat Med. 2021; 27(8):1385–1394. PMID: 34272499.30. Schäfer I, Oltrogge JH, Nestoriuc Y, Warren CV, Brassen S, Blattner M, et al. Expectations and prior experiences associated with adverse effects of COVID-19 vaccination. JAMA Netw Open. 2023; 6(3):e234732. PMID: 36972051.31. Alzarea AI, Khan YH, Alatawi AD, Alanazi AS, Alzarea SI, Butt MH, et al. Surveillance of post-vaccination side effects of COVID-19 vaccines among Saudi population: a real-world estimation of safety profile. Vaccines (Basel). 2022; 10(6):924. PMID: 35746532.32. Rahman MM, Masum MH, Wajed S, Talukder A. A comprehensive review on COVID-19 vaccines: development, effectiveness, adverse effects, distribution and challenges. Virusdisease. 2022; 33(1):1–22. PMID: 35127995.33. Abdel-Qader DH, Abdel-Qader H, Silverthorne J, Kongkaew C, Al Meslamani AZ, Hayajneh W, et al. Active safety surveillance of four types of COVID-19 vaccines: a national study from Jordan. Clin Drug Investig. 2022; 42(10):813–827.34. National Institutes of Health (NIH). COVID-19 immune response improves for months after vaccination. Updated 2022. Accessed May 29, 2024. https://www.nih.gov/news-events/nih-research-matters/covid-19-immune-response-improves-months-after-vaccination .35. Wisnewski AV, Campillo Luna J, Redlich CA. Human IgG and IgA responses to COVID-19 mRNA vaccines. PLoS One. 2021; 16(6):e0249499. PMID: 34133415.36. Ali HT, Ashour Y, Rais MA, Barakat M, Rezeq TA, Sharkawy MM, et al. Unravelling COVID-19 vaccination attributes worldwide: an extensive review regarding uptake, hesitancy, and future implication. Ann Med Surg (Lond). 2023; 85(7):3519–3530. PMID: 37427228.37. Vedhara K, Ayling K, Sunger K, Caldwell DM, Halliday V, Fairclough L, et al. Psychological interventions as vaccine adjuvants: a systematic review. Vaccine. 2019; 37(25):3255–3266. PMID: 31068258.38. Block JP, Boehmer TK, Forrest CB, Carton TW, Lee GM, Ajani UA, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022; 71(14):517–523. PMID: 35389977.39. Le Vu S, Bertrand M, Jabagi MJ, Botton J, Drouin J, Baricault B, et al. Age and sex-specific risks of myocarditis and pericarditis following COVID-19 messenger RNA vaccines. Nat Commun. 2022; 13(1):3633. PMID: 35752614.40. Mariotte E, Azoulay E, Galicier L, Rondeau E, Zouiti F, Boisseau P, et al. Epidemiology and pathophysiology of adulthood-onset thrombotic microangiopathy with severe ADAMTS13 deficiency (thrombotic thrombocytopenic purpura): a cross-sectional analysis of the French national registry for thrombotic microangiopathy. Lancet Haematol. 2016; 3(5):e237–e245. PMID: 27132698.41. Lee JS, Kim YH. Epidemiological trends of Bell’s palsy treated with steroids in Korea between 2008 and 2018. Muscle Nerve. 2021; 63(6):845–851. PMID: 33651414.42. Welsh KJ, Baumblatt J, Chege W, Goud R, Nair N. Thrombocytopenia including immune thrombocytopenia after receipt of mRNA COVID-19 vaccines reported to the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2021; 39(25):3329–3332. PMID: 34006408.43. Albakri K, Khaity A, Atwan H, Saleh O, Al-Hajali M, Cadri S, et al. Bell’s palsy and COVID-19 vaccines: a systematic review and meta-analysis. Vaccines (Basel). 2023; 11(2):236. PMID: 36851114.44. Yin A, Wang N, Shea PJ, Rosser EN, Kuo H, Shapiro JR, et al. Sex and gender differences in adverse events following influenza and COVID-19 vaccination. Biol Sex Differ. 2024; 15(1):50. PMID: 38890702.45. Flanagan KL, Fink AL, Plebanski M, Klein SL. Sex and gender differences in the outcomes of vaccination over the life course. Annu Rev Cell Dev Biol. 2017; 33(1):577–599. PMID: 28992436.46. Duijster JW, Lieber T, Pacelli S, Van Balveren L, Ruijs LS, Raethke M, et al. Sex-disaggregated outcomes of adverse events after COVID-19 vaccination: a Dutch cohort study and review of the literature. Front Immunol. 2023; 14:1078736. PMID: 36793715.47. Al-Qazaz HK, Al-Obaidy LM, Attash HM. COVID-19 vaccination, do women suffer from more side effects than men? A retrospective cross-sectional study. Pharm Pract (Granada). 2022; 20(2):2678. PMID: 35919795.48. Sharon NZ, Maymon R, Svirsky R, Novikov I, Cuckle H, Levtzion-Korach O. What do we know about abnormal uterine bleeding following vaccination against COVID-19 after two and a half years of experience? A systematic review and meta-analysis. Res Sq. 2024; 01. 01. DOI: 10.21203/rs.3.rs-3759326/v1.49. Pan American Health Organization. Consolidated regional and global information on adverse events following immunization (AEFI) against COVID-19 and other updates. Updated 2021. Accessed April, 2023. https://covid-19pharmacovigilance.paho.org/img/recursos/6183e3559c0bb8baa8f70f37e.pdf .50. Wang J, Tong Y, Li D, Li J, Li Y. The impact of age difference on the efficacy and safety of COVID-19 vaccines: a systematic review and meta-analysis. Front Immunol. 2021; 12:758294. PMID: 34938287.51. Alemayehu A, Demissie A, Yusuf M, Abdullahi Y, Abdulwehab R, Oljira L, et al. COVID-19 vaccine side effect: age and gender disparity in adverse effects following the first dose of AstraZeneca COVID-19 vaccine among the vaccinated population in Eastern Ethiopia: a community-based study. SAGE Open Med. 2022; 10:20503121221108616. PMID: 35832260.52. Ko M, Hwang I, Kim S, Kim H, Lee YK, Kwon Y. Monitoring status of adverse events following immunization on the third dose of the COVID-19 vaccine. Public Health Wkly Rep. 2022; 15(2):82–90.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- COVID-19 vaccine safety monitoring in Republic of Korea from February 26, 2021 to October 31, 2021
- Adverse events and preventive measures related to COVID-19 vaccines
- Adverse Events Following Immunization Associated with Coronavirus Disease 2019 Vaccination Reported in the Mobile Vaccine Adverse Events Reporting System
- Adverse events following vaccination against coronavirus disease 2019
- Adverse Events in Healthcare Workers after the First Dose of ChAdOx1 nCoV-19 or BNT162b2 mRNA COVID-19 Vaccination: a Single Center Experience