J Korean Med Sci.
2024 Nov;39(42):e275. 10.3346/jkms.2024.39.e275.
Genome-Wide Association Analysis of Rapid Decline in Lung Function: Analysis From the Korean Genome and Epidemiology Study
- Affiliations
-
- 1Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Internal Medicine, Dongguk University Gyeongju Hospital, Dongguk University College of Medicine, Gyeongju, Korea
- 2Department of Internal Medicine, Hanyang University College of Medicine, Seoul, Korea
- 3Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 4Department of Laboratory Medicine, Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 5Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2560620
- DOI: http://doi.org/10.3346/jkms.2024.39.e275
Abstract
- Background
A rapid decline in forced expiratory volume in 1 second (FEV1) is considered an important phenotype of the development of chronic obstructive pulmonary disease (COPD). However, the associations between specific genetic variants (single-nucleotide polymorphisms; SNPs) and this phenotype remain uncertain.
Methods
We enrolled 6,516 individuals from the Korean Genome and Epidemiology Study (KoGES). A rapid decline in FEV1 was defined as an annual decrease of FEV1 ≥ 60 mL/year. A multivariable logistic regression model was used to assess the associations between SNP variants and the rapid decline in FEV1. Considering the significant impact of smoking on lung function, a subgroup analysis based on smoking history was also conducted.
Results
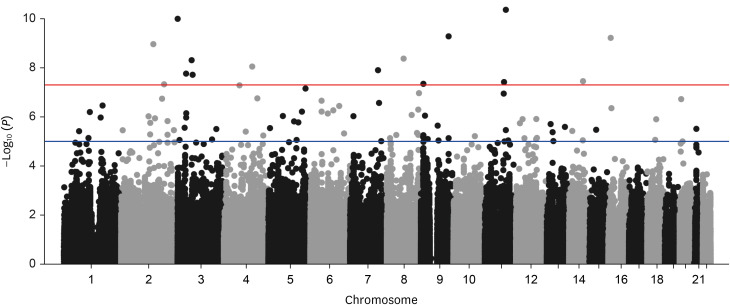
A genome-wide association analysis of the rapid decline in FEV1 identified 15 association signals (P < 5.0 × 10−8 ). Among the 15 nucleotide variants, rs9833533 and rs1496255 have been previously reported to be associated with lung function development. In the subgroup analysis, rs16951883 (adjusted odds ratio [aOR], 3.24; P = 5.87 × 10−8 ) was the most significant SNP associated with rapid decline in FEV1 among never smokers, followed by rs41476549, rs16840064, and rs1350110. Conversely, among ever smokers, rs10959478 (aOR, 4.74; P = 8.27 × 10−7 ) showed the highest significance, followed by rs6805861, rs9833533, and rs16906215.
Conclusion
We identified 15 nucleotide variants linked to a rapid decline in FEV1, including two SNPs previously reported to be associated with lung function development. Additional SNPs, which were associated with COPD, may be found using novel phenotypes.
Keyword
Figure
Reference
-
1. Christenson SA, Smith BM, Bafadhel M, Putcha N. Chronic obstructive pulmonary disease. Lancet. 2022; 399(10342):2227–2242. PMID: 35533707.2. Decramer M, Rennard S, Troosters T, Mapel DW, Giardino N, Mannino D, et al. COPD as a lung disease with systemic consequences--clinical impact, mechanisms, and potential for early intervention. COPD. 2008; 5(4):235–256. PMID: 18671149.3. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for prevention, diagnosis and management of COPD: 2024 report. Updated 2024. Accessed July 27, 2024. http://goldcopd.org/2024-gold-report .4. Kim SH, Lee H, Joo H, Choi H, Sim YS, Rhee CK, et al. Risk of rapid lung function decline in young adults with chronic obstructive pulmonary disease: a community-based prospective cohort study. J Korean Med Sci. 2023; 38(1):e3. PMID: 36593687.5. Whittaker HR, Bloom C, Morgan A, Jarvis D, Kiddle SJ, Quint JK. Accelerated FEV1 decline and risk of cardiovascular disease and mortality in a primary care population of COPD patients. Eur Respir J. 2021; 57(3):2000918. PMID: 32972984.6. Lee HW, Lee HJ, Lee JK, Park TY, Heo EY, Kim DK. Rapid FEV1 decline and lung cancer incidence in South Korea. Chest. 2022; 162(2):466–474. PMID: 35318007.7. Kemp R, Pustulka I, Boerner G, Smela B, Hofstetter E, Sabeva Y, et al. Relationship between FEV1 decline and mortality in patients with bronchiolitis obliterans syndrome-a systematic literature review. Respir Med. 2021; 188:106608. PMID: 34517199.8. de Serres F, Blanco I. Role of alpha-1 antitrypsin in human health and disease. J Intern Med. 2014; 276(4):311–335. PMID: 24661570.9. Silverman EK. Genetics of COPD. Annu Rev Physiol. 2020; 82:413–431. PMID: 31730394.10. Nam K, Kim J, Lee S. Genome-wide study on 72,298 individuals in Korean biobank data for 76 traits. Cell Genom. 2022; 2(10):100189. PMID: 36777999.11. Wilk JB, Chen TH, Gottlieb DJ, Walter RE, Nagle MW, Brandler BJ, et al. A genome-wide association study of pulmonary function measures in the Framingham Heart Study. PLoS Genet. 2009; 5(3):e1000429. PMID: 19300500.12. Suh Y, Lee C. Genome-wide association study for genetic variants related with maximal voluntary ventilation reveals two novel genomic signals associated with lung function. Medicine (Baltimore). 2017; 96(44):e8530. PMID: 29095316.13. Martinez FJ, Han MK, Allinson JP, Barr RG, Boucher RC, Calverley PM, et al. At the root: defining and halting progression of early chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018; 197(12):1540–1551. PMID: 29406779.14. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005; 26(2):319–338. PMID: 16055882.15. Kim SH, Kim HS, Min HK, Lee SW. Association between insulin resistance and lung function trajectory over 4 years in South Korea: community-based prospective cohort. BMC Pulm Med. 2021; 21(1):110. PMID: 33794844.16. Jeon S, Shin JY, Yee J, Park T, Park M. Structural equation modeling for hypertension and type 2 diabetes based on multiple SNPs and multiple phenotypes. PLoS One. 2019; 14(9):e0217189. PMID: 31513605.17. Ong BA, Li J, McDonough JM, Wei Z, Kim C, Chiavacci R, et al. Gene network analysis in a pediatric cohort identifies novel lung function genes. PLoS One. 2013; 8(9):e72899. PMID: 24023788.18. Sin DD. The importance of early chronic obstructive pulmonary disease: a lecture from 2022 Asian Pacific Society of Respirology. Tuberc Respir Dis (Seoul). 2023; 86(2):71–81. PMID: 37005090.19. Stolz D, Mkorombindo T, Schumann DM, Agusti A, Ash SY, Bafadhel M, et al. Towards the elimination of chronic obstructive pulmonary disease: a Lancet Commission. Lancet. 2022; 400(10356):921–972. PMID: 36075255.20. Fawcett KA, Song K, Qian G, Farmaki AE, Packer R, John C, et al. Pleiotropic associations of heterozygosity for the SERPINA1 Z allele in the UK Biobank. ERJ Open Res. 2021; 7(2):00049-2021. PMID: 33981765.21. Sin DD. Chronic obstructive pulmonary disease and the airway microbiome: what respirologists need to know. Tuberc Respir Dis (Seoul). 2023; 86(3):166–175. PMID: 37038880.22. Sircar K, Hnizdo E, Petsonk E, Attfield M. Decline in lung function and mortality: implications for medical monitoring. Occup Environ Med. 2007; 64(7):461–466. PMID: 17332137.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Short History of the Genome-Wide Association Study: Where We Were and Where We Are Going
- Genome-Wide Association Study of Hepatitis in Korean Populations
- Genome-Wide Architecture of East Asian Patients With Migraine: A Genome-Wide Association Study Based on Familial History
- Gene Set Analyses of Genome-Wide Association Studies on 49 Quantitative Traits Measured in a Single Genetic Epidemiology Dataset
- Introduction of Bioinformatic Methods for the Gene Function Analysis