J Yeungnam Med Sci.
2024 Oct;41(4):269-278. 10.12701/jyms.2024.00752.
Screening and treatment of endocrine hypertension focusing on adrenal gland disorders: a narrative review
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Yeungnam University College of Medicine, Daegu, Korea
- KMID: 2560487
- DOI: http://doi.org/10.12701/jyms.2024.00752
Abstract
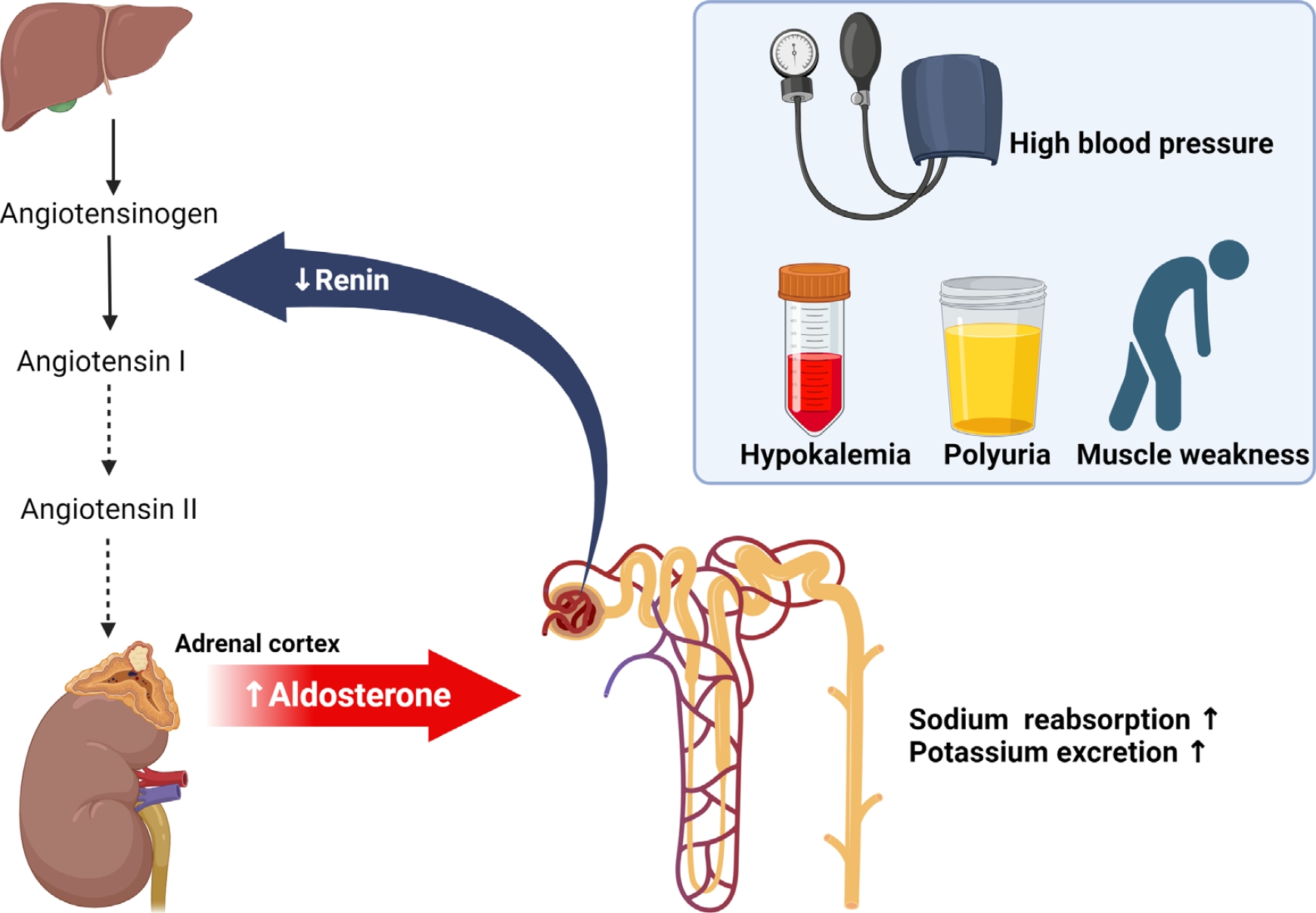
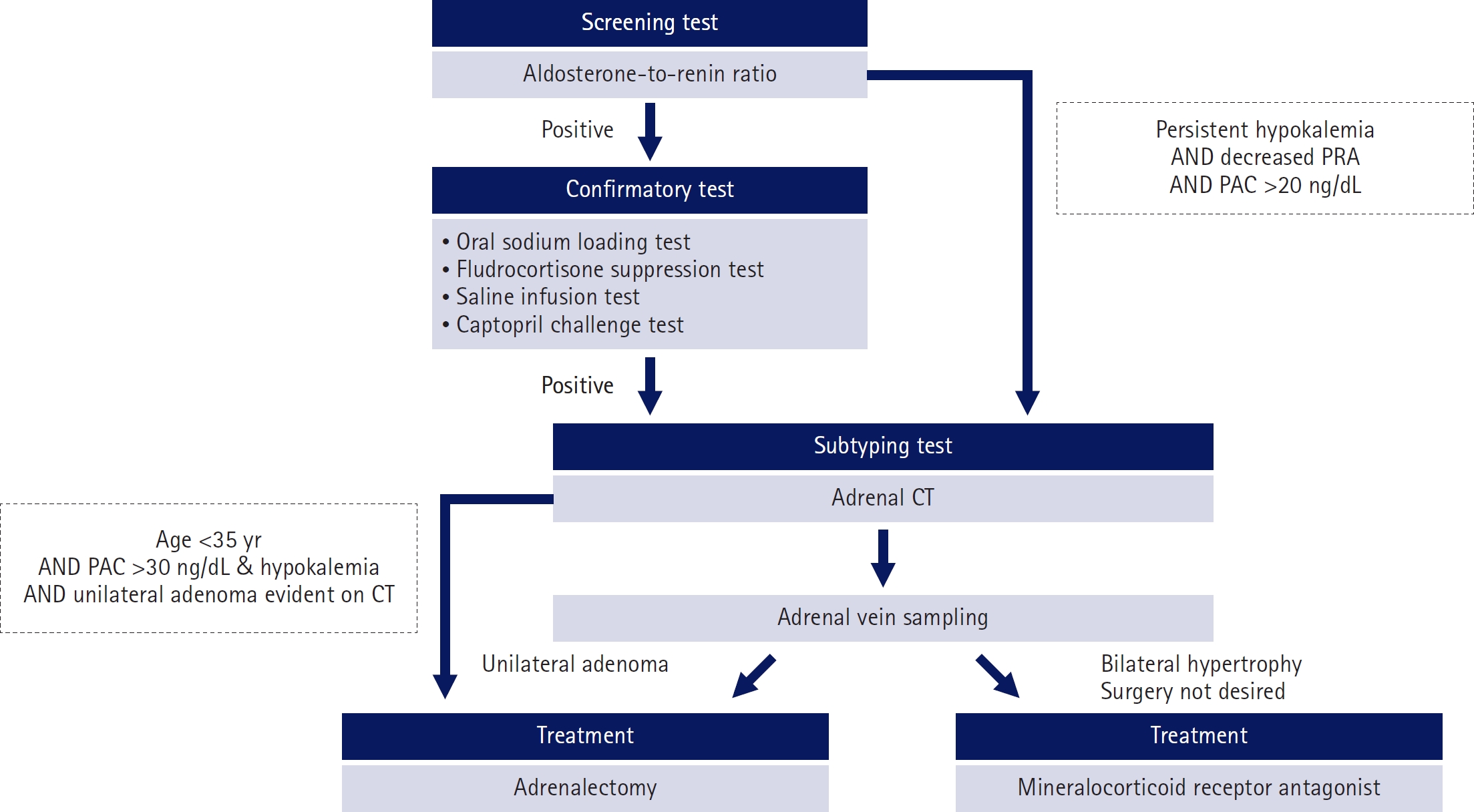
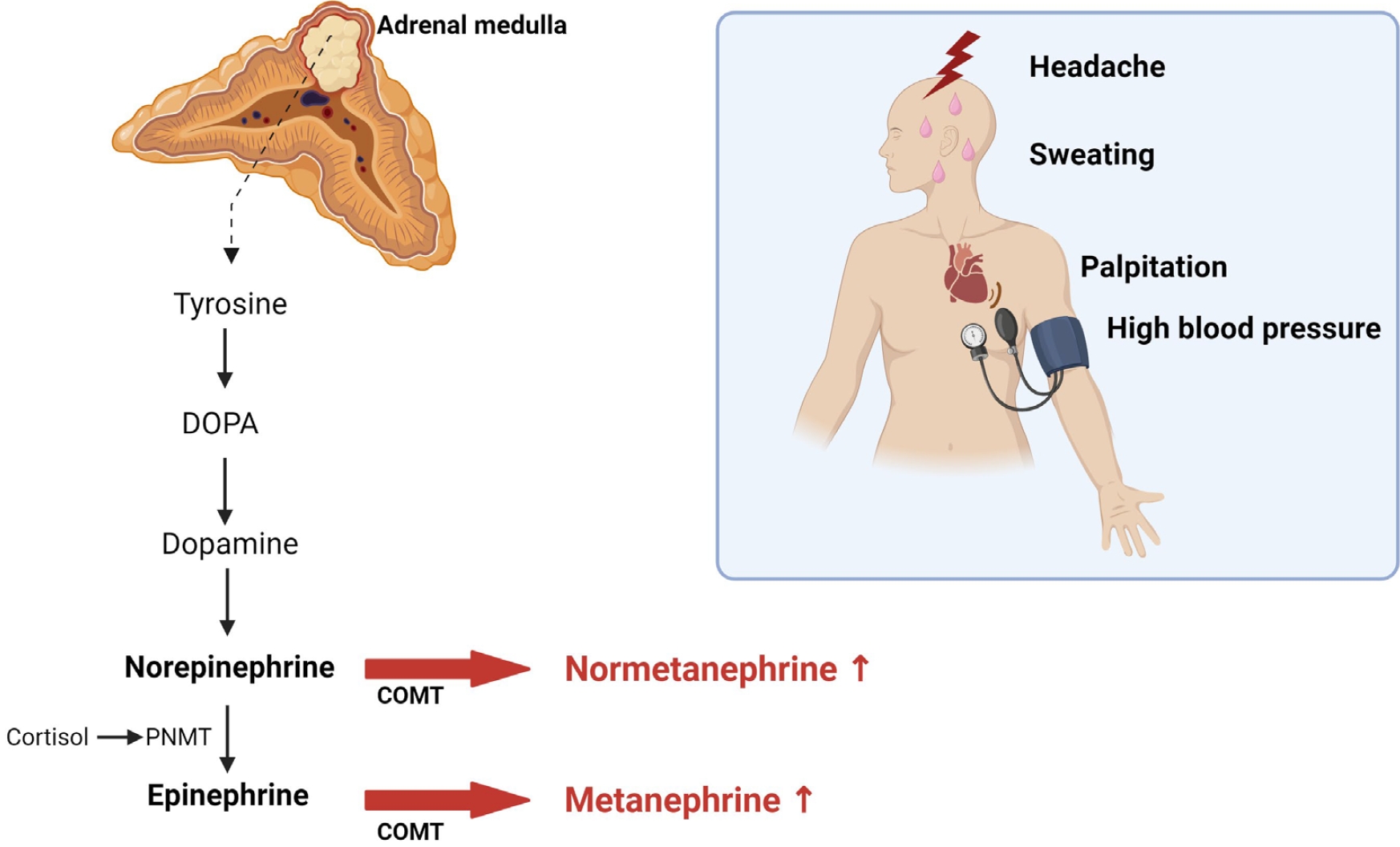
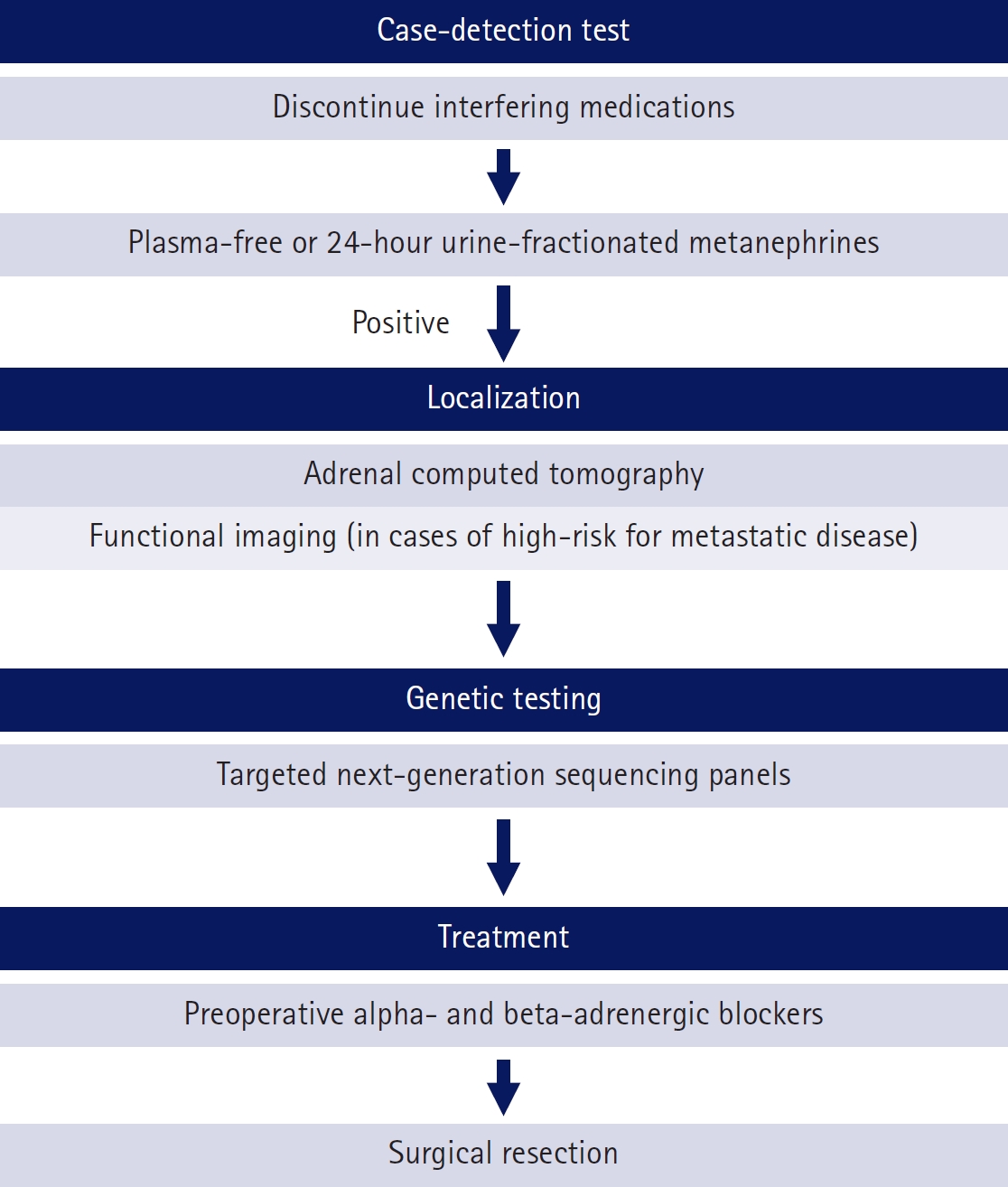
- Most cases of high blood pressure have no identifiable cause, termed essential hypertension; however, in approximately 15% of cases, hypertension occurs due to secondary causes. Primary aldosteronism (PA) and pheochromocytoma and paraganglioma (PPGL) are representative endocrine hypertensive diseases. The differentiation of endocrine hypertension provides an opportunity to cure and prevent target organ damage. PA is the most common cause of secondary hypertension, which significantly increases the risk of cardiovascular disease compared to essential hypertension; thus, patients with clinical manifestations suggestive of secondary hypertension should be screened for PA. PPGL are rare but can be fatal when misdiagnosed. PPGL are the most common hereditary endocrine tumors; therefore, genetic testing using next-generation sequencing panels is recommended. Herein, we aimed to summarize the characteristic clinical symptoms of PA and PPGL and when and how diagnostic tests and treatment strategies should be performed.
Figure
Reference
-
References
1. Korean Society of Hypertension, Hypertension Epidemiology Research Working Group. Korea hypertension fact sheet 2022 [Internet]. Seoul: Korean Society of Hypertension; 2022 [cited 2024 Jul 15]. https://drive.google.com/file/d/1s2OROEH_OaUyI-h97DvAhOGP-oUtn2nX/view?usp=sharing.2. Young Jr WF, Calhoun DA, Lenders JW, Stowasser M, Textor SC. Screening for endocrine hypertension: an endocrine society scientific statement. Endocr Rev. 2017; 38:103–22.3. Kotchen TA. Hypertension. In : Loscalzo J, Fauci A, Kasper D, Hauser S, Longo D, Jameson JL, editors. Harrison’s principles of internal medicine. 21st ed. New York, NY: McGraw-Hill Education;2022.4. Rimoldi SF, Scherrer U, Messerli FH. Secondary arterial hypertension: when, who, and how to screen? Eur Heart J. 2014; 35:1245–54.5. Young WF Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019; 285:126–48.6. Monticone S, Burrello J, Tizzani D, Bertello C, Viola A, Buffolo F, et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. 2017; 69:1811–20.7. Mulatero P, Stowasser M, Loh KC, Fardella CE, Gordon RD, Mosso L, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004; 89:1045–50.8. Mulatero P, Monticone S, Burrello J, Veglio F, Williams TA, Funder J. Guidelines for primary aldosteronism: uptake by primary care physicians in Europe. J Hypertens. 2016; 34:2253–7.9. Choy KW, Fuller PJ, Russell G, Li Q, Leenaerts M, Yang J. Primary aldosteronism. BMJ. 2022; 377:e065250.10. Vaidya A, Mulatero P, Baudrand R, Adler GK. The expanding spectrum of primary aldosteronism: implications for diagnosis, pathogenesis, and treatment. Endocr Rev. 2018; 39:1057–88.11. Monticone S, D’Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018; 6:41–50.12. Burrello J, Monticone S, Losano I, Cavaglià G, Buffolo F, Tetti M, et al. Prevalence of hypokalemia and primary aldosteronism in 5100 patients referred to a tertiary hypertension unit. Hypertension. 2020; 75:1025–33.13. Ha J, Park JH, Kim KJ, Kim JH, Jung KY, Lee J, et al. 2023 Korean Endocrine Society consensus guidelines for the diagnosis and management of primary aldosteronism. Endocrinol Metab (Seoul). 2023; 38:597–618.14. Mulatero P, Monticone S, Deinum J, Amar L, Prejbisz A, Zennaro MC, et al. Genetics, prevalence, screening and confirmation of primary aldosteronism: a position statement and consensus of the Working Group on Endocrine Hypertension of The European Society of Hypertension. J Hypertens. 2020; 38:1919–28.15. Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016; 101:1889–916.16. Whelton PK, Carey RM, Mancia G, Kreutz R, Bundy JD, Williams B. Harmonization of the American College of Cardiology/American Heart Association and European Society of Cardiology/European Society of Hypertension Blood Pressure/Hypertension Guidelines. Eur Heart J. 2022; 43:3302–11.17. Kim HL, Lee EM, Ahn SY, Kim KI, Kim HC, Kim JH, et al. The 2022 focused update of the 2018 Korean Hypertension Society Guidelines for the management of hypertension. Clin Hypertens. 2023; 29:11.18. Dudenbostel T, Calhoun DA. Resistant hypertension, obstructive sleep apnoea and aldosterone. J Hum Hypertens. 2012; 26:281–7.19. Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005; 45:1243–8.20. Seccia TM, Letizia C, Muiesan ML, Lerco S, Cesari M, Bisogni V, et al. Atrial fibrillation as presenting sign of primary aldosteronism: results of the Prospective Appraisal on the Prevalence of Primary Aldosteronism in Hypertensive (PAPPHY) Study. J Hypertens. 2020; 38:332–9.21. Halimi JM, Mimran A. Albuminuria in untreated patients with primary aldosteronism or essential hypertension. J Hypertens. 1995; 13(12 Pt 2):1801–2.22. Notsu M, Yamauchi M, Yamamoto M, Nawata K, Sugimoto T. Primary aldosteronism as a risk factor for vertebral fracture. J Clin Endocrinol Metab. 2017; 102:1237–43.23. Monticone S, Sconfienza E, D’Ascenzo F, Buffolo F, Satoh F, Sechi LA, et al. Renal damage in primary aldosteronism: a systematic review and meta-analysis. J Hypertens. 2020; 38:3–12.24. Faconti L, Kulkarni S, Delles C, Kapil V, Lewis P, Glover M, et al. Diagnosis and management of primary hyperaldosteronism in patients with hypertension: a practical approach endorsed by the British and Irish Hypertension Society. J Hum Hypertens. 2024; 38:8–18.25. Stowasser M, Ahmed AH, Cowley D, Wolley M, Guo Z, McWhinney BC, et al. Comparison of seated with recumbent saline suppression testing for the diagnosis of primary aldosteronism. J Clin Endocrinol Metab. 2018; 103:4113–24.26. Kwon SY, Park J, Park SH, Cho SH, Lee YB, Lee SY, et al. Aldosterone immunoassay-specific cutoff value for seated saline suppression test for diagnosing primary aldosteronism. Endocrinol Metab (Seoul). 2022; 37:938–42.27. Thuzar M, Young K, Ahmed AH, Ward G, Wolley M, Guo Z, et al. Diagnosis of primary aldosteronism by seated saline suppression test-variability between immunoassay and HPLC-MS/MS. J Clin Endocrinol Metab. 2020; 105:e477–83.28. Luther JM, Turcu AF. Unilaterally successful adrenal vein sampling: use or repeat? Hypertension. 2023; 80:2014–6.29. Mulatero P, Tizzani D, Viola A, Bertello C, Monticone S, Mengozzi G, et al. Prevalence and characteristics of familial hyperaldosteronism: the PATOGEN study (Primary Aldosteronism in TOrino-GENetic forms). Hypertension. 2011; 58:797–803.30. Tetti M, Brüdgam D, Burrello J, Udager AM, Riester A, Knösel T, et al. Unilateral primary aldosteronism: long-term disease recurrence after adrenalectomy. Hypertension. 2024; 81:936–45.31. Harris DA, Au-Yong I, Basnyat PS, Sadler GP, Wheeler MH. Review of surgical management of aldosterone secreting tumours of the adrenal cortex. Eur J Surg Oncol. 2003; 29:467–74.32. Rossi GP, Cesari M, Cuspidi C, Maiolino G, Cicala MV, Bisogni V, et al. Long-term control of arterial hypertension and regression of left ventricular hypertrophy with treatment of primary aldosteronism. Hypertension. 2013; 62:62–9.33. Fourkiotis V, Vonend O, Diederich S, Fischer E, Lang K, Endres S, et al. Effectiveness of eplerenone or spironolactone treatment in preserving renal function in primary aldosteronism. Eur J Endocrinol. 2012; 168:75–81.34. Williams TA, Lenders JW, Mulatero P, Burrello J, Rottenkolber M, Adolf C, et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017; 5:689–99.35. Ghose RP, Hall PM, Bravo EL. Medical management of aldosterone-producing adenomas. Ann Intern Med. 1999; 131:105–8.36. Cohen JB, Bancos I, Brown JM, Sarathy H, Turcu AF, Cohen DL. Primary aldosteronism and the role of mineralocorticoid receptor antagonists for the heart and kidneys. Annu Rev Med. 2023; 74:217–30.37. Sehgal R, Singh H, Singh IP. Comparative study of spironolactone and eplerenone in management of ascites in patients of cirrhosis of liver. Eur J Gastroenterol Hepatol. 2020; 32:535–9.38. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999; 341:709–17.39. de Gasparo M, Whitebread SE, Preiswerk G, Jeunemaître X, Corvol P, Ménard J. Antialdosterones: incidence and prevention of sexual side effects. J Steroid Biochem. 1989; 32(1B):223–7.40. Kolkhof P, Delbeck M, Kretschmer A, Steinke W, Hartmann E, Bärfacker L, et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J Cardiovasc Pharmacol. 2014; 64:69–78.41. Dojki FK, Bakris G. Nonsteroidal mineralocorticoid antagonists in diabetic kidney disease. Curr Opin Nephrol Hypertens. 2017; 26:368–74.42. Li Q. Efficacy and safety of finerenone in patients with primary aldosteronism. ClinicalTrials.gov ID: NCT05924620 [Internet]. Bethesda, MD: National Library of Medicine;2024 [cited 2024 Jul 19]. https://clinicaltrials.gov/study/NCT05924620.43. Li Q. Finerenone for Patients With Primary Aldosteronism (FAIRY). ClinicalTrials.gov ID: NCT06457074 [Internet]. Bethesda, MD: National Library of Medicine;2024 [cited 2024 Jul 19]. https://clinicaltrials.gov/study/NCT06457074.44. Carey RM, Calhoun DA, Bakris GL, Brook RD, Daugherty SL, Dennison-Himmelfarb CR, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018; 72:e53–90.45. Kantorovich V, Pacak K. Pheochromocytoma and paraganglioma. Prog Brain Res. 2010; 182:343–73.46. Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014; 99:1915–42.47. Eisenhofer G. Screening for pheochromocytomas and paragangliomas. Curr Hypertens Rep. 2012; 14:130–7.48. Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005; 366:665–75.49. Canu L, Parenti G, De Filpo G, Mannelli M. Pheochromocytomas and paragangliomas as causes of endocrine hypertension. Front Endocrinol (Lausanne). 2019; 10:333.50. Melson E, Amir S, Shepherd L, Kauser S, Freestone B, Kempegowda P. Myocardial Infarction with non-obstructed coronaries: atypical presentation of pheochromocytoma. Endocrinol Diabetes Metab Case Rep. 2019; 2019:EDM190089.51. Lee YY, Chung SM. Interleukin-6-producing paraganglioma as a rare cause of systemic inflammatory response syndrome: a case report. J Yeungnam Med Sci. 2023; 40:435–41.52. Gruber LM, Hartman RP, Thompson GB, McKenzie TJ, Lyden ML, Dy BM, et al. Pheochromocytoma characteristics and behavior differ depending on method of discovery. J Clin Endocrinol Metab. 2019; 104:1386–93.53. Young WF Jr. Clinical presentation and diagnosis of pheochromocytoma [Internet]. Waltham, MA: UpToDate;2024 [cited 2024 Jul 19]. https://www.uptodate.com/contents/clinical-presentation-and-diagnosis-of-pheochromocytoma.54. Eisenhofer G, Keiser H, Friberg P, Mezey E, Huynh TT, Hiremagalur B, et al. Plasma metanephrines are markers of pheochromocytoma produced by catechol-O-methyltransferase within tumors. J Clin Endocrinol Metab. 1998; 83:2175–85.55. Peaston RT, Graham KS, Chambers E, van der Molen JC, Ball S. Performance of plasma free metanephrines measured by liquid chromatography-tandem mass spectrometry in the diagnosis of pheochromocytoma. Clin Chim Acta. 2010; 411:546–52.56. Mullins F, O’Shea P, FitzGerald R, Tormey W. Enzyme-linked immunoassay for plasma-free metanephrines in the biochemical diagnosis of phaeochromocytoma in adults is not ideal. Clin Chem Lab Med. 2011; 50:105–10.57. Neary NM, King KS, Pacak K. Drugs and pheochromocytoma: don’t be fooled by every elevated metanephrine. N Engl J Med. 2011; 364:2268–70.58. Leow MK, Loh KC, Kiat Kwek T, Ng PY. Catecholamine and metanephrine excess in intracerebral haemorrhage: revisiting an obscure yet common “pseudophaeochromocytoma”. J Clin Pathol. 2007; 60:583–4.59. Grouzmann E, Drouard-Troalen L, Baudin E, Plouin PF, Muller B, Grand D, et al. Diagnostic accuracy of free and total metanephrines in plasma and fractionated metanephrines in urine of patients with pheochromocytoma. Eur J Endocrinol. 2010; 162:951–60.60. Sawka AM, Jaeschke R, Singh RJ, Young WF Jr. A comparison of biochemical tests for pheochromocytoma: measurement of fractionated plasma metanephrines compared with the combination of 24-hour urinary metanephrines and catecholamines. J Clin Endocrinol Metab. 2003; 88:553–8.61. Bravo EL. Evolving concepts in the pathophysiology, diagnosis, and treatment of pheochromocytoma. Endocr Rev. 1994; 15:356–68.62. Canu L, Van Hemert JA, Kerstens MN, Hartman RP, Khanna A, Kraljevic I, et al. CT Characteristics of pheochromocytoma: relevance for the evaluation of adrenal incidentaloma. J Clin Endocrinol Metab. 2019; 104:312–8.63. Ku EJ, Kim KJ, Kim JH, Kim MK, Ahn CH, Lee KA, et al. Diagnosis for pheochromocytoma and paraganglioma: a joint position statement of the Korean Pheochromocytoma and Paraganglioma Task Force. Endocrinol Metab (Seoul). 2021; 36:322–38.64. Neumann HP, Young WF Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019; 381:552–65.65. Muth A, Crona J, Gimm O, Elmgren A, Filipsson K, Stenmark Askmalm M, et al. Genetic testing and surveillance guidelines in hereditary pheochromocytoma and paraganglioma. J Intern Med. 2019; 285:187–204.66. Fang F, Ding L, He Q, Liu M. Preoperative management of pheochromocytoma and paraganglioma. Front Endocrinol (Lausanne). 2020; 11:586795.67. Lenders JW, Kerstens MN, Amar L, Prejbisz A, Robledo M, Taieb D, et al. Genetics, diagnosis, management and future directions of research of phaeochromocytoma and paraganglioma: a position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J Hypertens. 2020; 38:1443–56.68. Sibal L, Jovanovic A, Agarwal SC, Peaston RT, James RA, Lennard TW, et al. Phaeochromocytomas presenting as acute crises after beta blockade therapy. Clin Endocrinol (Oxf). 2006; 65:186–90.69. Wang L, Zeng W, Wu Y, Gong Z. Comparison of clinical efficacy and safety between robotic-assisted and laparoscopic adrenalectomy for pheochromocytoma: a systematic review and meta-analysis. J Robot Surg. 2024; 18:115.70. Ilanchezhian M, Jha A, Pacak K, Del Rivero J. Emerging treatments for advanced/metastatic pheochromocytoma and paraganglioma. Curr Treat Options Oncol. 2020; 21:85.71. FDA approves AZEDRA specified use in pheochromocytomas/paragangliomas. J Nucl Med. 2018; 59:17N.72. Pryma DA, Chin BB, Noto RB, Dillon JS, Perkins S, Solnes L, et al. Efficacy and safety of high-specific-activity 131I-MIBG therapy in patients with advanced pheochromocytoma or paraganglioma. J Nucl Med. 2019; 60:623–30.73. Thorpe MP, Kane A, Zhu J, Morse MA, Wong T, Borges-Neto S. Long-term outcomes of 125 patients with metastatic pheochromocytoma or paraganglioma treated with 131-I MIBG. J Clin Endocrinol Metab. 2020; 105:e494–501.74. O’Kane GM, Ezzat S, Joshua AM, Bourdeau I, Leibowitz-Amit R, Olney HJ, et al. A phase 2 trial of sunitinib in patients with progressive paraganglioma or pheochromocytoma: the SNIPP trial. Br J Cancer. 2019; 120:1113–9.75. Baudin E, Goichot B, Berruti A, Hadoux J, Moalla S, Laboureau S, et al. Sunitinib for metastatic progressive phaeochromocytomas and paragangliomas: results from FIRSTMAPPP, an academic, multicentre, international, randomised, placebo-controlled, double-blind, phase 2 trial. Lancet. 2024; 403:1061–70.76. Plouin PF, Amar L, Dekkers OM, Fassnacht M, Gimenez-Roqueplo AP, Lenders JW, et al. European Society of Endocrinology Clinical Practice Guideline for long-term follow-up of patients operated on for a phaeochromocytoma or a paraganglioma. Eur J Endocrinol. 2016; 174:G1–10.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pediatric Endocrine Hypertension Related to the Adrenal Glands
- Endocrine Disorders in the Sick and Preterm Newborn
- Overview of endocrine tumor syndromes manifesting as adrenal tumors
- The Overview of Endocrine Hypertension
- Bilateral adrenal adenomas with autonomous cortisol secretion from both glands and autonomous aldosterone secretion from the left adrenal: a case report