Intest Res.
2024 Oct;22(4):484-495. 10.5217/ir.2024.00021.
Perianal fistulizing lesions of Crohn’s disease are associated with long-term behavior and its transition: a Chinese cohort study
- Affiliations
-
- 1Department of Gastroenterology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- KMID: 2560298
- DOI: http://doi.org/10.5217/ir.2024.00021
Abstract
- Background/Aims
Crohn’s disease (CD) has a progressive nature and commonly perianal involvement. The aim of this study is to assess the prevalence, surgical treatment, and outcome of perianal fistulizing CD with associated risk factors in a large Chinese cohort.
Methods
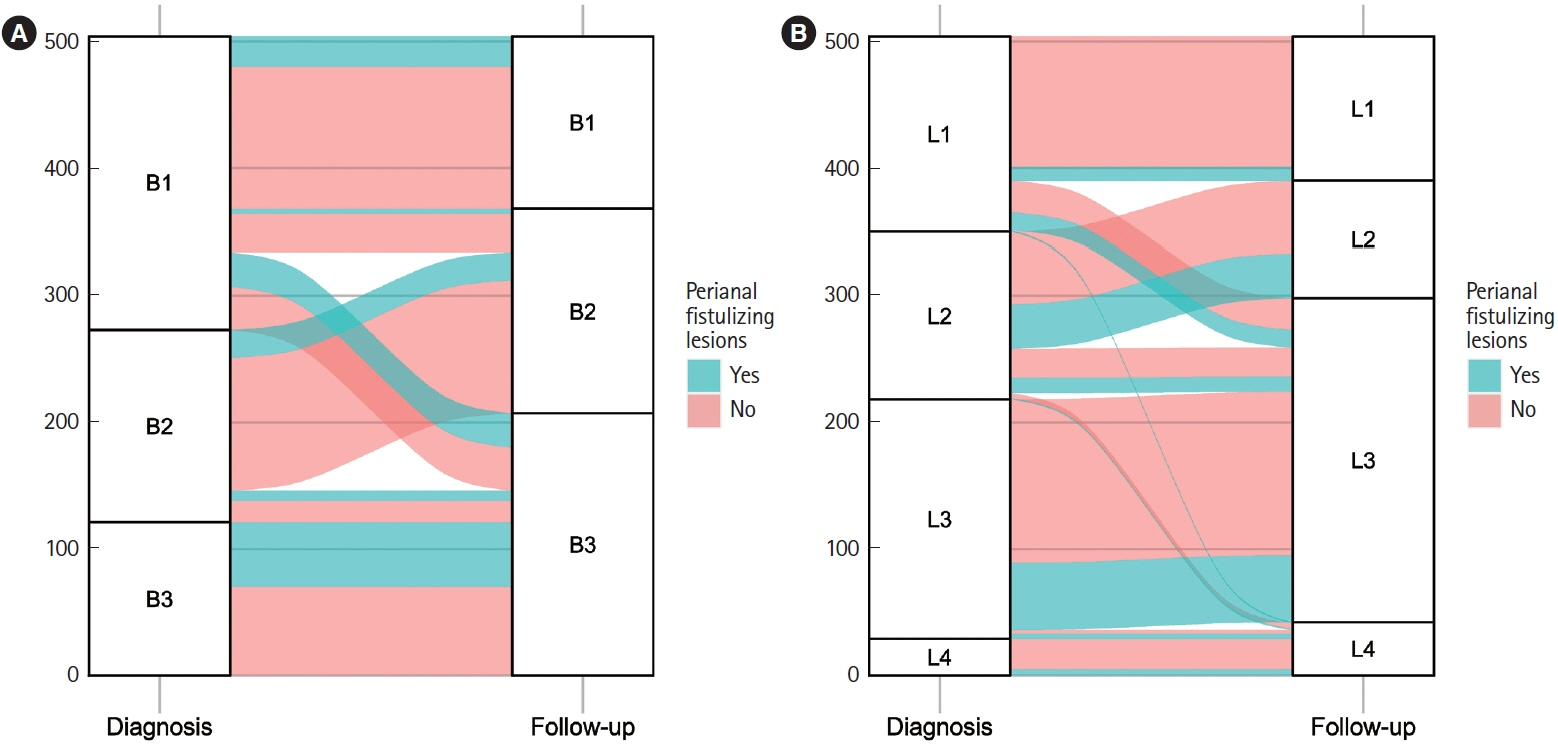
Hospitalized patients diagnosed with CD in our center were consecutively enrolled between January 2000 and December 2018. Transition of disease behavior was classified according to the presence or absence of penetrating behavior (B3 in the Montreal classification) at diagnosis and at a median follow-up of 102 months.
Results
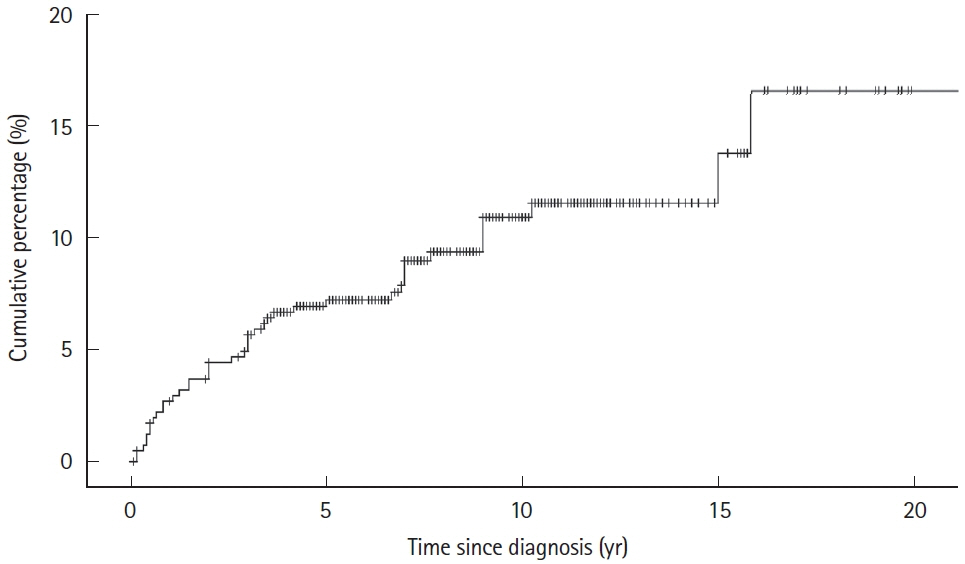
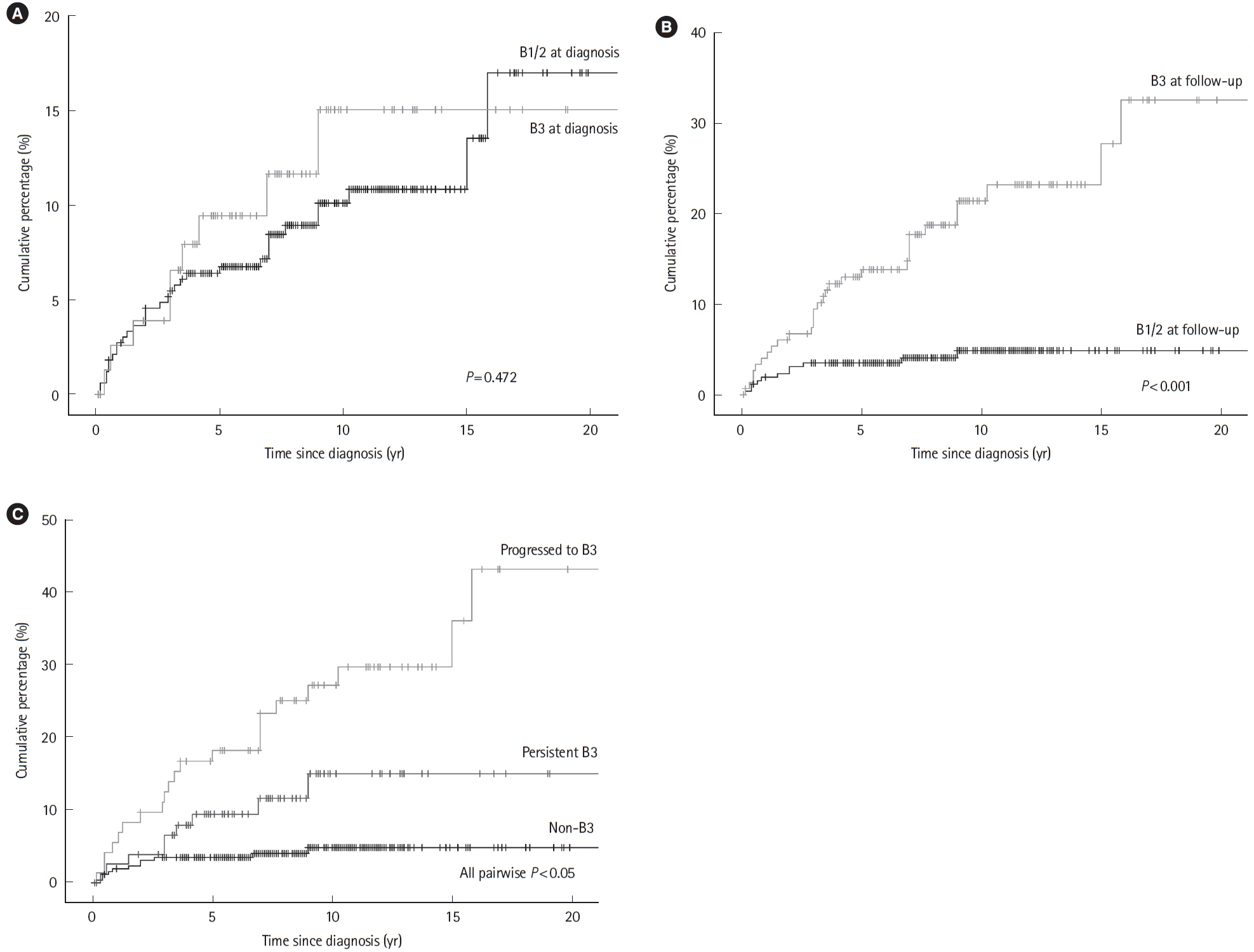
A total of 504 patients were included, of whom 207 (41.1%) were classified as B3 and 348 (69.0%) as L2/3 at follow-up. Transition of behavior to B3 was observed in 86 patients (17.1%). The incidence of perianal fistulizing lesions was 10.9% at 10 years with a final prevalence of 27.0% (n = 136) at the end of follow-up. Multivariate Cox regression identified independent risks of perianal fistulizing lesions for persistent B3 (hazard ratio, 4.72; 95% confidence interval, 1.91–11.66) and behavior transition of progressed to B3 (hazard ratio, 9.90; 95% confidence interval, 4.60–21.33). Perianal surgical treatments were performed in 104 patients (20.6%). Thirty-six cases (7.1%) were refractory, and it is independently associated with behavior of persistent B3 (P= 0.011).
Conclusions
Perianal fistulizing lesions occurred frequently in Chinese CD patients. Its incidence and refractory outcome were closely associated with the penetrating CD behavior. An additional risk of perianal fistulizing lesions was indicated for CD patients with behavior of progressing to B3, suggesting further attention.
Figure
Reference
-
1. Burisch J, Kiudelis G, Kupcinskas L, et al. Natural disease course of Crohn’s disease during the first 5 years after diagnosis in a European population-based inception cohort: an Epi-IBD study. Gut. 2019; 68:423–433.2. Hughes LE. Clinical classification of perianal Crohn’s disease. Dis Colon Rectum. 1992; 35:928–932.
Article3. Mahadev S, Young JM, Selby W, Solomon MJ. Quality of life in perianal Crohn’s disease: what do patients consider important? Dis Colon Rectum. 2011; 54:579–585.
Article4. Kasparek MS, Glatzle J, Temeltcheva T, Mueller MH, Koenigsrainer A, Kreis ME. Long-term quality of life in patients with Crohn’s disease and perianal fistulas: influence of fecal diversion. Dis Colon Rectum. 2007; 50:2067–2074.
Article5. Panés J, Rimola J. Perianal fistulizing Crohn’s disease: pathogenesis, diagnosis and therapy. Nat Rev Gastroenterol Hepatol. 2017; 14:652–664.6. Tsai L, McCurdy JD, Ma C, Jairath V, Singh S. Epidemiology and natural history of perianal Crohn’s disease: a systematic review and meta-analysis of population-based cohorts. Inflamm Bowel Dis. 2022; 28:1477–1484.
Article7. Schwartz DA, Loftus EV, Tremaine WJ, et al. The natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology. 2002; 122:875–880.
Article8. Eglinton TW, Barclay ML, Gearry RB, Frizelle FA. The spectrum of perianal Crohn’s disease in a population-based cohort. Dis Colon Rectum. 2012; 55:773–777.
Article9. Ng WK, Wong SH, Ng SC. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res. 2016; 14:111–119.
Article10. Zeng Z, Zhu Z, Yang Y, et al. Incidence and clinical characteristics of inflammatory bowel disease in a developed region of Guangdong Province, China: a prospective population-based study. J Gastroenterol Hepatol. 2013; 28:1148–1153.11. Song EM, Lee HS, Kim YJ, et al. Incidence and outcomes of perianal disease in an Asian population with Crohn’s disease: a nationwide population-based study. Dig Dis Sci. 2020; 65:1189–1196.
Article12. Jangi S, Ruan A, Korzenik J, de Silva P. South Asian patients with inflammatory bowel disease in the United States demonstrate more fistulizing and perianal Crohn phenotype. Inflamm Bowel Dis. 2020; 26:1933–1942.
Article13. Kaplan GG, Ng SC. Globalisation of inflammatory bowel disease: perspectives from the evolution of inflammatory bowel disease in the UK and China. Lancet Gastroenterol Hepatol. 2016; 1:307–316.
Article14. Kennedy NA, Jones GR, Plevris N, Patenden R, Arnott ID, Lees CW. Association between level of fecal calprotectin and progression of Crohn’s disease. Clin Gastroenterol Hepatol. 2019; 17:2269–2276.
Article15. Plevris N, Fulforth J, Lyons M, et al. Normalization of fecal calprotectin within 12 months of diagnosis is associated with reduced risk of disease progression in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2021; 19:1835–1844.16. Bai X, Zhang H, Ruan G, et al. Long-term disease behavior and surgical intervention analysis in hospitalized patients with Crohn’s disease in China: a retrospective cohort study. Inflamm Bowel Dis. 2022; 28(Suppl 2):S35–S41.
Article17. Inflammatory Bowel Disease Group; Chinese Society of Gastroenterology; Chinese Medical Association. Chinese consensus on diagnosis and treatment in inflammatory bowel disease (2018, Beijing). J Dig Dis. 2021; 22:298–317.18. Gomollón F, Dignass A, Annese V, et al. 3rd European evidencebased consensus on the diagnosis and management of Crohn’s disease 2016: part 1. Diagnosis and medical management. J Crohns Colitis. 2017; 11:3–25.
Article19. Best WR. Predicting the Crohn’s disease activity index from the Harvey-Bradshaw Index. Inflamm Bowel Dis. 2006; 12:304–310.
Article20. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006; 55:749–753.
Article21. Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004; 60:505–512.22. Mak WY, Mak OS, Lee CK, et al. Significant medical and surgical morbidity in perianal Crohn’s disease: results from a territory-wide study. J Crohns Colitis. 2018; 12:1392–1398.
Article23. Yamamoto T, Nakase H, Watanabe K, et al. Diagnosis and clinical features of perianal lesions in newly diagnosed Crohn’s disease: subgroup analysis from inception cohort registry study of patients with Crohn’s disease (iCREST-CD). J Crohns Colitis. 2023; 17:1193–1206.
Article24. Göttgens KW, Jeuring SF, Sturkenboom R, et al. Time trends in the epidemiology and outcome of perianal fistulizing Crohn’s disease in a population-based cohort. Eur J Gastroenterol Hepatol. 2017; 29:595–601.
Article25. Wils P, Leroyer A, Fumery M, Fernandez-Nistal A, Gower-Rousseau C, Pariente B. Fistulizing perianal lesions in a French population with Crohn’s disease. Dig Liver Dis. 2021; 53:661–665.
Article26. Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002; 8:244–250.27. Tang LY, Rawsthorne P, Bernstein CN. Are perineal and luminal fistulas associated in Crohn’s disease? A population-based study. Clin Gastroenterol Hepatol. 2006; 4:1130–1134.
Article28. Sachar DB, Bodian CA, Goldstein ES, et al. Is perianal Crohn’s disease associated with intestinal fistulization? Am J Gastroenterol. 2005; 100:1547–1549.
Article29. Tarrant KM, Barclay ML, Frampton CM, Gearry RB. Perianal disease predicts changes in Crohn’s disease phenotype-results of a population-based study of inflammatory bowel disease phenotype. Am J Gastroenterol. 2008; 103:3082–3093.
Article30. Penner RM, Madsen KL, Fedorak RN. Postoperative Crohn’s disease. Inflamm Bowel Dis. 2005; 11:765–777.
Article31. Bernstein CN, Loftus EV Jr, Ng SC, Lakatos PL, Moum B; Epidemiology and Natural History Task Force of the International Organization for the Study of Inflammatory Bowel Disease (IOIBD). Hospitalisations and surgery in Crohn’s disease. Gut. 2012; 61:622–629.32. Heuman R, Bolin T, Sjödahl R, Tagesson C. The incidence and course of perianal complications and arthralgia after intestinal resection with restoration of continuity for Crohn’s disease. Br J Surg. 1981; 68:528–530.
Article33. Sandborn WJ, Fazio VW, Feagan BG, Hanauer SB; American Gastroenterological Association Clinical Practice Committee. AGA technical review on perianal Crohn’s disease. Gastroenterology. 2003; 125:1508–1530.
Article34. Lee MJ, Parker CE, Taylor SR, et al. Efficacy of medical therapies for fistulizing Crohn’s disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018; 16:1879–1892.
Article35. Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med. 1999; 340:1398–1405.
Article36. Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004; 350:876–885.37. Shehab M, Alrashed F, Heron V, Restellini S, Bessissow T. Comparative efficacy of biologic therapies for inducing response and remission in fistulizing Crohn’s disease: systematic review and network meta-analysis of randomized controlled trials. Inflamm Bowel Dis. 2023; 29:367–375.
Article38. Qiu Y, Chen BL, Mao R, et al. Systematic review with meta-analysis: loss of response and requirement of anti-TNFα dose intensification in Crohn’s disease. J Gastroenterol. 2017; 52:535–554.
Article39. Wewer MD, Zhao M, Nordholm-Carstensen A, Weimers P, Seidelin JB, Burisch J. The incidence and disease course of perianal Crohn’s disease: a Danish nationwide cohort study, 1997-2015. J Crohns Colitis. 2021; 15:5–13.
Article40. Sica GS, Di Carlo S, Tema G, et al. Treatment of peri-anal fistula in Crohn’s disease. World J Gastroenterol. 2014; 20:13205–13210.
Article41. Graf W, Andersson M, Åkerlund JE, Börjesson L; Swedish Organization for Studies of Inflammatory Bowel Disease. Longterm outcome after surgery for Crohn’s anal fistula. Colorectal Dis. 2016; 18:80–85.42. Kusaka J, Shiga H, Kuroha M, et al. Risk factors associated with postoperative recurrence and repeat surgery in Japanese patients with Crohn’s disease. Int J Colorectal Dis. 2017; 32:1407–1413.
Article43. Buisson A, Chevaux JB, Allen PB, Bommelaer G, Peyrin-Biroulet L. Review article: the natural history of postoperative Crohn’s disease recurrence. Aliment Pharmacol Ther. 2012; 35:625–633.
Article44. Nam K, Jung WB, Lee SB, Soh JS, Yang SS, Jung SW. Predictors of reoperation for perianal fistula in Crohn’s disease. J Dig Dis. 2021; 22:334–341.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Incidentally Detected Asymptomatic Perianal Abscess in an Adolescent during Crohn's Disease Diagnosis: Is Routine Pelvic Imaging Required in Korean Pediatric Patients at Diagnosis?
- Self-screening questionnaire for perianal fistulizing disease in patients with Crohn’s disease
- Vedolizumab for perianal fistulizing Crohn’s disease: systematic review and meta-analysis
- A Case of Crohn's Disease Showing a Skin Lesion with a Cobblestone-like Appearance in the Perianal Region
- Surgical treatment of perianal fistula in Crohn's disease