Cancer Res Treat.
2024 Oct;56(4):1164-1170. 10.4143/crt.2024.009.
Association between Endoscopist Volume and Interval Cancers after Colonoscopy: Results from the National Colorectal Cancer Screening Program in Korea
- Affiliations
-
- 1Department of Preventive Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Department of Public Health, Graduate School, The Catholic University of Korea, Seoul, Korea
- 3Division of Social Welfare and Health Administration, Wonkwang University, Iksan, Korea
- 4National Cancer Control Institute, National Cancer Center, Goyang, Korea
- 5Graduate School of Cancer Science and Policy, National Cancer Center, Goyang, Korea
- 6Department of Public Health and Healthcare Management, Graduate School, The Catholic University of Korea, Seoul, Korea
- 7Department of Medical Science, Soonchunhyang University Graduate School, Asan, Korea
- KMID: 2560250
- DOI: http://doi.org/10.4143/crt.2024.009
Abstract
- Purpose
The rate of interval colorectal cancer (iCRC) is now accepted as a key performance indicator of organized colorectal cancer (CRC) screening programs. We aimed to examine the association between endoscopist volumes and the rate of iCRC among individuals with a positive fecal immunochemical test (FIT) within a nationwide population-based CRC screening program.
Materials and Methods
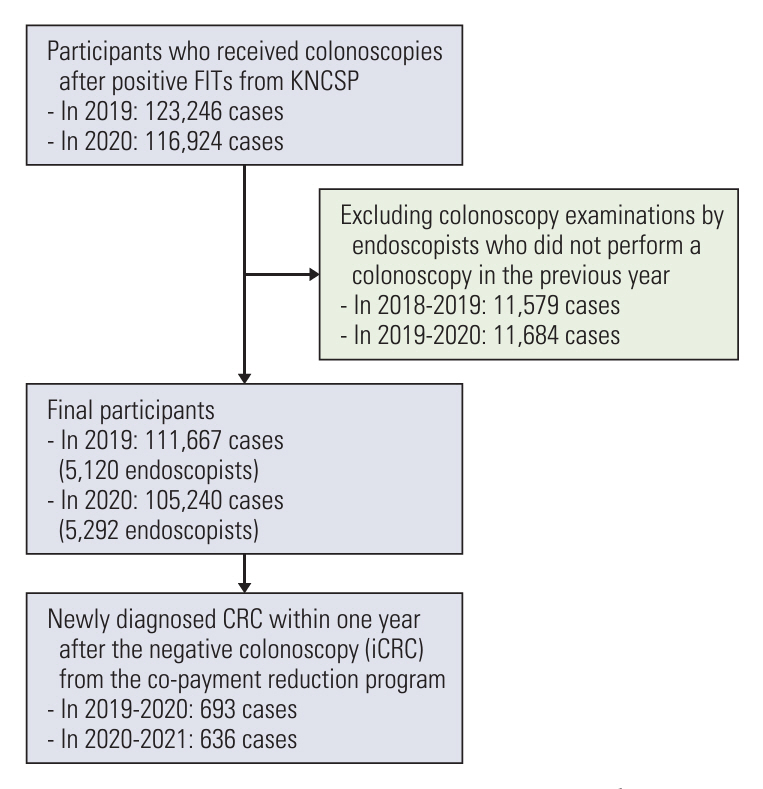
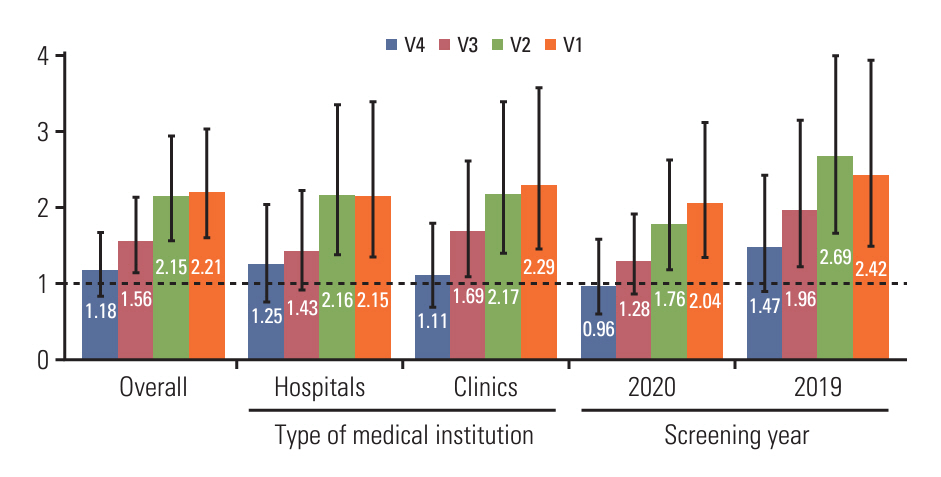
Individuals aged ≥ 50 years who underwent colonoscopy after a positive FIT from January 1, 2019 until December 31, 2020 in the Korean National Cancer Screening Program (KNCSP) were enrolled. We converted the data into per-endoscopist screening results, calculated the iCRC rates per endoscopist, and compared them to the previous year’s annual volume that was divided into five groups (V1, 1-9; V2, 10-29; V3, 30-59; V4, 60-119; V5, ≥ 120).
Results
A total of 10,412 endoscopists performed 216,907 colonoscopies. Overall, the average rate of iCRC per endoscopist was 8.46 per 1,000 examinations. Compared with the group with the highest volume (V5 group), the rate of iCRC was 2.21 times higher in the V1 group. Similar trends were observed in the other groups (V2: relative risks [RR], 2.15; 95% confidence interval [CI], 1.57 to 2.94; V3: RR, 1.56; 95% CI, 1.15 to 2.13; V4: RR, 1.18; 95% CI, 0.83 to 1.67).
Conclusion
The findings emphasize that endoscopists with lower procedure volumes have higher risks of interval cancer being missed or undetected. To maximize the preventative impact of colonoscopy for CRC, this issue should be addressed by monitoring endoscopist volumes and variations in performances.
Keyword
Figure
Reference
-
References
1. Onyoh EF, Hsu WF, Chang LC, Lee YC, Wu MS, Chiu HM. The rise of colorectal cancer in Asia: epidemiology, screening, and management. Curr Gastroenterol Rep. 2019; 21:36.
Article2. Sung JJ, Lau JY, Goh KL, Leung WK; Asia Pacific Working Group on Colorectal Cancer. Increasing incidence of colorectal cancer in Asia: implications for screening. Lancet Oncol. 2005; 6:871–6.
Article3. Park B, Lee YY, Song SY, Shin HY, Suh M, Choi KS, et al. Trends of colorectal cancer screening rates in Korea: Korean National Cancer Screening Survey 2005-2020. Gut Liver. 2022; 16:930–41.
Article4. Kuipers EJ, Rosch T, Bretthauer M. Colorectal cancer screening: optimizing current strategies and new directions. Nat Rev Clin Oncol. 2013; 10:130–42.
Article5. Shin A, Choi KS, Jun JK, Noh DK, Suh M, Jung KW, et al. Validity of fecal occult blood test in the national cancer screening program, Korea. PLoS One. 2013; 8:e79292.
Article6. Sanduleanu S, Masclee AM, Meijer GA. Interval cancers after colonoscopy-insights and recommendations. Nat Rev Gastroenterol Hepatol. 2012; 9:550–4.
Article7. Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010; 362:1795–803.
Article8. Brenner H, Chang-Claude J, Seiler CM, Hoffmeister M. Interval cancers after negative colonoscopy: population-based case-control study. Gut. 2012; 61:1576–82.
Article9. Hassan C, Rex DK, Zullo A, Cooper GS. Loss of efficacy and cost-effectiveness when screening colonoscopy is performed by nongastroenterologists. Cancer. 2012; 118:4404–11.
Article10. Mehta A, Efron DT, Canner JK, Dultz L, Xu T, Jones C, et al. Effect of surgeon and hospital volume on emergency general surgery outcomes. J Am Coll Surg. 2017; 225:666–75.
Article11. Forbes N, Boyne DJ, Mazurek MS, Hilsden RJ, Sutherland RL, Pader J, et al. Association between endoscopist annual procedure Volume and colonoscopy quality: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2020; 18:2192–208.
Article12. Lee K, Lee YY, Suh M, Jun JK, Park B, Kim Y, et al. Impact of COVID-19 on cancer screening in South Korea. Sci Rep. 2022; 12:11380.
Article13. Sanduleanu S, le Clercq CM, Dekker E, Meijer GA, Rabeneck L, Rutter MD, et al. Definition and taxonomy of interval colorectal cancers: a proposal for standardising nomenclature. Gut. 2015; 64:1257–67.
Article14. Ertem FU, Ladabaum U, Mehrotra A, Tehranian S, Shi Z, Saul M, et al. Incidence of interval colorectal cancer attributable to an endoscopist in clinical practice. Gastrointest Endosc. 2018; 88:705–11.
Article15. Park B, Her EY, Lee K, Nari F, Jun JK, Choi KS, et al. Overview of the national cancer screening program for colorectal cancer in Korea over 14 years (2004-2017). Cancer Res Treat. 2023; 55:910–7.
Article16. Lee CK, Choi KS, Eun CS, Park DI, Han DS, Yoon M, et al. Risk and characteristics of postcolonoscopy interval colorectal cancer after a positive fecal test: a nationwide populationbased study in Korea. Cancer Res Treat. 2018; 50:50–9.
Article17. Shin DW, Cho J, Park JH, Cho B. National general health screening program in Korea: history, current status, and future direction: a scoping review. Precis Future Med. 2022; 6:9–31.
Article18. Chen W, Shi J, Qian L, Azen SP. Comparison of robustness to outliers between robust poisson models and log-binomial models when estimating relative risks for common binary outcomes: a simulation study. BMC Med Res Methodol. 2014; 14:82.
Article19. Wisse PH, Erler NS, de Boer SY, den Hartog B, Oudkerk Pool M, Terhaar Sive Droste JS, et al. Adenoma detection rate and risk for interval postcolonoscopy colorectal cancer in fecal immunochemical test-based screening: a population-based cohort study. Ann Intern Med. 2022; 175:1366–73.
Article20. Chiu SY, Chuang SL, Chen SL, Yen AM, Fann JC, Chang DC, et al. Faecal haemoglobin concentration influences risk prediction of interval cancers resulting from inadequate colonoscopy quality: analysis of the Taiwanese Nationwide Colorectal Cancer Screening Program. Gut. 2017; 66:293–300.
Article21. Gotfried J, Bernstein M, Ehrlich AC, Friedenberg FK. Administrative database research overestimates the rate of interval colon cancer. J Clin Gastroenterol. 2015; 49:483–90.
Article22. Cooper GS, Xu F, Barnholtz Sloan JS, Schluchter MD, Koroukian SM. Prevalence and predictors of interval colorectal cancers in medicare beneficiaries. Cancer. 2012; 118:3044–52.
Article23. Singh H, Nugent Z, Demers AA, Bernstein CN. Rate and predictors of early/missed colorectal cancers after colonoscopy in Manitoba: a population-based study. Am J Gastroenterol. 2010; 105:2588–96.
Article24. Cha JM. Colonoscopy quality is the answer for the emerging issue of interval cancer. Intest Res. 2014; 12:110–6.
Article25. Adler J, Robertson DJ. Interval colorectal cancer after colonoscopy: exploring explanations and solutions. Am J Gastroenterol. 2015; 110:1657–64.
Article26. Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003; 349:2117–27.
Article27. Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002; 346:1128–37.
Article28. Yoon JY, Cha JM, Jeen YT; Medical Policy Committee of Korean Association for the Study of Intestinal Diseases (KASID); Quality Improvement Committee of Korean Society of Gastrointestinal Endoscopy (KSGE). Quality is the key for emerging issues of population-based colonoscopy screening. Clin Endosc. 2018; 51:50–5.
Article29. Tinmouth J, Kennedy EB, Baron D, Burke M, Feinberg S, Gould M, et al. Colonoscopy quality assurance in Ontario: systematic review and clinical practice guideline. Can J Gastroenterol Hepatol. 2014; 28:251–74.
Article30. Valori R, Rey JF, Atkin WS, Bretthauer M, Senore C, Hoff G, et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis, first edition: quality assurance in endoscopy in colorectal cancer screening and diagnosis. Endoscopy. 2012; 44 Suppl 3:SE88–105.31. Min JK, Cha JM, Cho YK, Kim JH, Yoon SM, Im JP, et al. Revision of quality indicators for the endoscopy quality improvement program of the national cancer screening program in Korea. Clin Endosc. 2018; 51:239–52.
Article32. Hilsden RJ, Rose SM, Dube C, Rostom A, Bridges R, McGregor SE, et al. Defining and applying locally relevant benchmarks for the adenoma detection rate. Am J Gastroenterol. 2019; 114:1315–21.
Article33. Jover R, Zapater P, Bujanda L, Hernandez V, Cubiella J, Pellise M, et al. Endoscopist characteristics that influence the quality of colonoscopy. Endoscopy. 2016; 48:241–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Colonoscopy Quality is the Answer for the Emerging Issue of Interval Cancer
- Training in Endoscopy: Colonoscopy
- Clinical and Biological Features of Interval Colorectal Cancer
- Colon Cancer Screening with Image-Enhanced Endoscopy
- Korean National Recommendation Guidelines on Screening and Surveillance for Early Detection of Colorectal Cancers