Cancer Res Treat.
2024 Oct;56(4):1068-1076. 10.4143/crt.2024.008.
Analysis of Response and Progression Patterns of Tyrosine Kinase Inhibitors in Recurrent or Metastatic Adenoid Cystic Carcinoma: A Post Hoc Analysis of Two KCSG Phase II Trials
- Affiliations
-
- 1Department of Hematology and Oncology, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea
- 2Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- 3Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea
- 4Division of Hematology-Oncology, Department of Internal Medicine, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
- 5Department of Internal Medicine, SMG-SNU Boramae Medical Center, Seoul, Korea
- 6Department of Internal Medicine, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea
- 7Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 8Division of Hematology-Oncology, Department of Internal Medicine, Chungnam National University College of Medicine, Daejeon, Korea
- 9Ewha Womans University Medical Center, Seoul, Korea
- 10Department of Internal Medicine, Chungbuk University Hospital, Chungbuk University College of Medicine, Cheongju, Korea
- 11Division of Hematology-Oncology, Department of Internal Medicine, Dongnam Institute of Radiological and Medical Sciences, Busan, Korea
- 12Division of Hematology-Oncology, Department of Internal Medicine, Yeungnam University Hospital, Yeungnam University College of Medicine, Daegu, Korea
- 13Division of Hematology-Oncology, Department of Internal Medicine, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea
- 14Rare Cancers Clinic, Center for Specific Organs Cancer, National Cancer Center, Goyang, Korea
- 15Department of Internal Medicine, Busan Paik Hospital, Inje University College of Medicine, Busan, Korea
- 16Division of Oncology, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 17Division of Hematology-Oncology, Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2560241
- DOI: http://doi.org/10.4143/crt.2024.008
Abstract
- Purpose
In this study, we evaluated 66 patients diagnosed with adenoid cystic carcinoma (ACC) enrolled in two Korean Cancer Study Group trials to investigate the response and progression patterns in recurrent and/or metastatic ACC treated with vascular endothelial growth factor receptor tyrosine kinase inhibitors (VEGFR-TKIs).
Materials and Methods
We evaluated 66 patients diagnosed with ACC who were enrolled in the Korean Cancer Study Group trials. The tumor measurements, clinical data, treatment outcomes, and progression patterns of therapy were analyzed.
Results
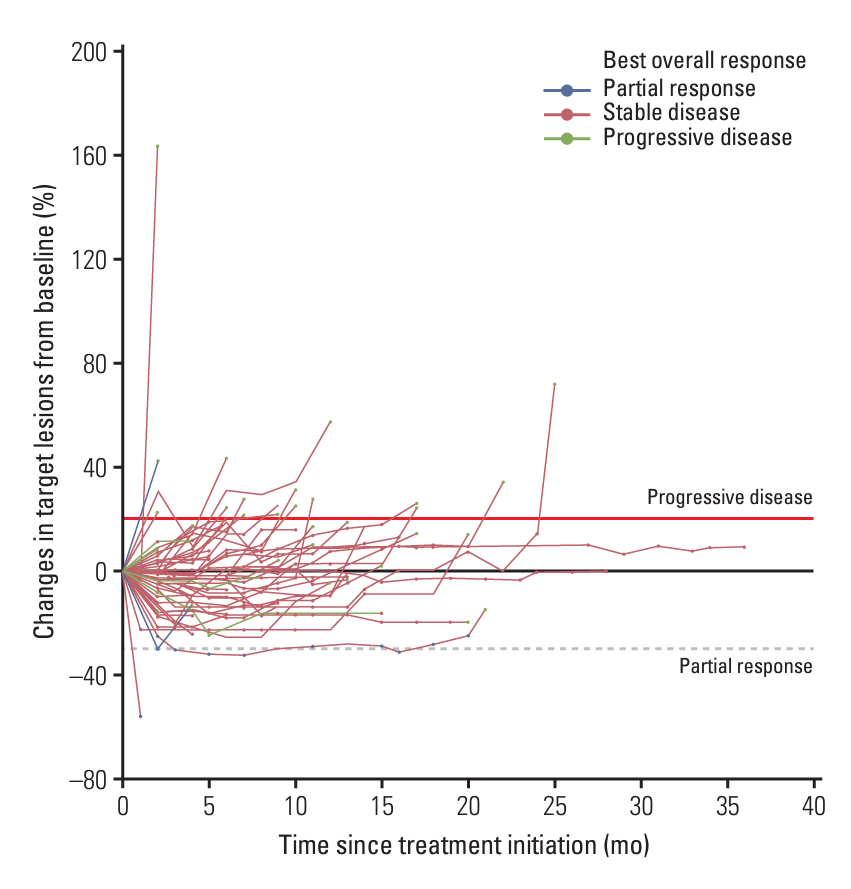
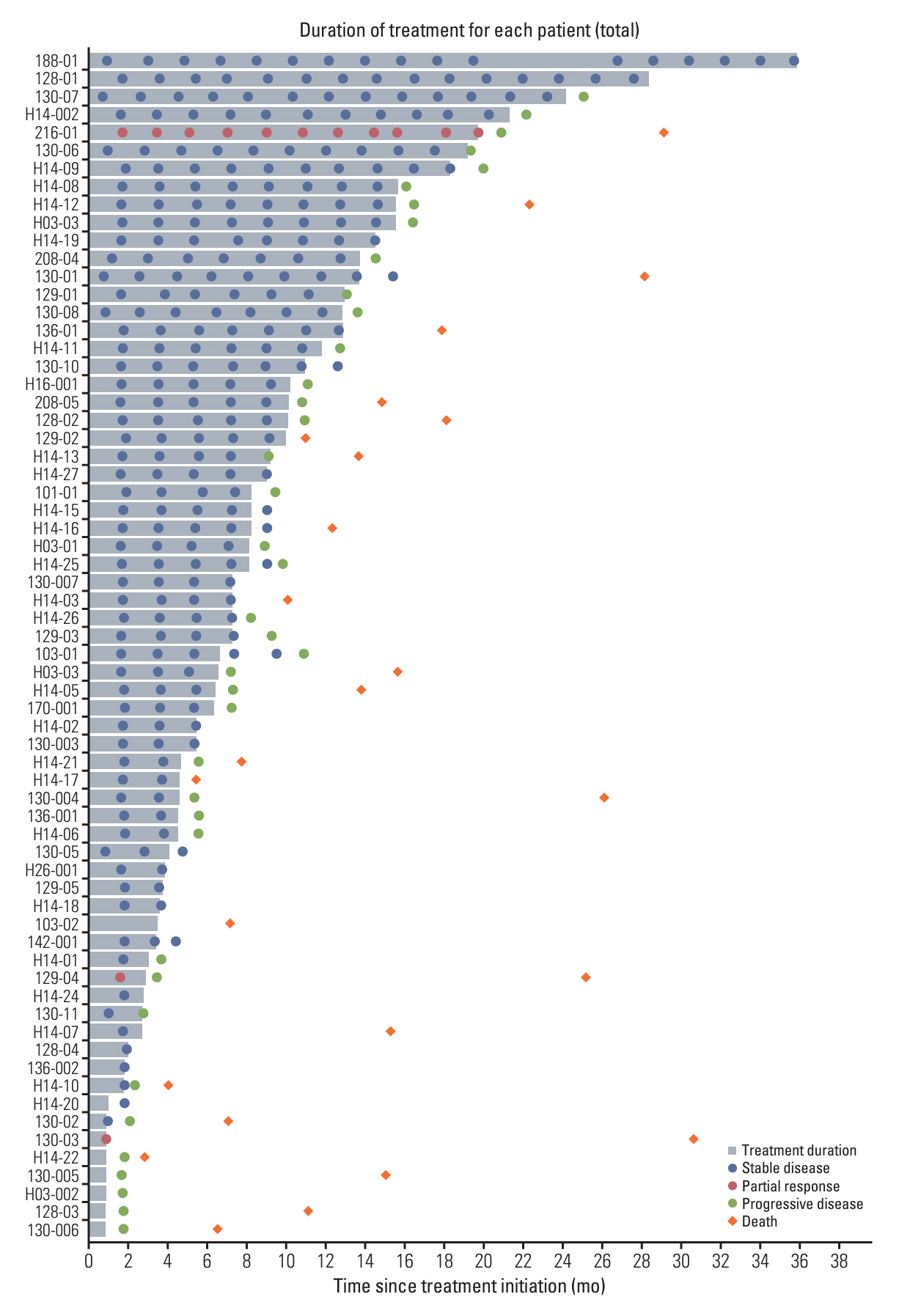
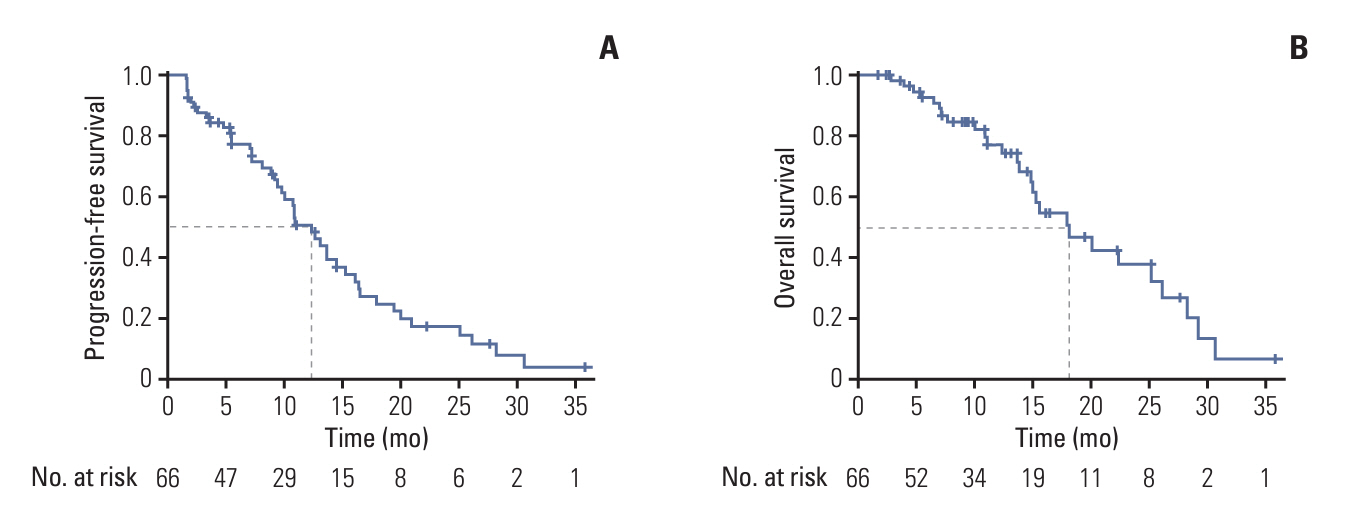
In the 66 patients (53 receiving axitinib and 13 receiving nintedanib), the disease control rate was 61%, and three patients achieved partial response. The median follow-up, median progression-free survival (PFS), overall survival, and 6-month PFS rate were 27.6%, 12.4%, and 18.1% months and 62.1%, respectively. Among 42 patients who experienced progression, 27 (64.3%) showed target lesion progression. Bone metastasis was an independent poor prognostic factor.
Conclusion
Overall, most patients demonstrated stable disease with prolonged PFS; however, prominent target lesion progression occurred in some patients. Thus, PFS may capture VEGFR-TKI efficacy better than the objective response rate.
Keyword
Figure
Reference
-
References
1. Laurie SA, Ho AL, Fury MG, Sherman E, Pfister DG. Systemic therapy in the management of metastatic or locally recurrent adenoid cystic carcinoma of the salivary glands: a systematic review. Lancet Oncol. 2011; 12:815–24.
Article2. Sung MW, Kim KH, Kim JW, Min YG, Seong WJ, Roh JL, et al. Clinicopathologic predictors and impact of distant metastasis from adenoid cystic carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 2003; 129:1193–7.
Article3. Park S, Nam SJ, Keam B, Kim TM, Jeon YK, Lee SH, et al. VEGF and Ki-67 overexpression in predicting poor overall survival in adenoid cystic carcinoma. Cancer Res Treat. 2016; 48:518–26.
Article4. Tchekmedyian V, Sherman EJ, Dunn L, Tran C, Baxi S, Katabi N, et al. Phase II study of lenvatinib in patients with progressive, recurrent or metastatic adenoid cystic carcinoma. J Clin Oncol. 2019; 37:1529–37.
Article5. Chau NG, Hotte SJ, Chen EX, Chin SF, Turner S, Wang L, et al. A phase II study of sunitinib in recurrent and/or metastatic adenoid cystic carcinoma (ACC) of the salivary glands: current progress and challenges in evaluating molecularly targeted agents in ACC. Ann Oncol. 2012; 23:1562–70.
Article6. Thomson DJ, Silva P, Denton K, Bonington S, Mak SK, Swindell R, et al. Phase II trial of sorafenib in advanced salivary adenoid cystic carcinoma of the head and neck. Head Neck. 2015; 37:182–7.
Article7. Kim Y, Lee SJ, Lee JY, Lee SH, Sun JM, Park K, et al. Clinical trial of nintedanib in patients with recurrent or metastatic salivary gland cancer of the head and neck: a multicenter phase 2 study (Korean Cancer Study Group HN14-01). Cancer. 2017; 123:1958–64.
Article8. Keam B, Kim SB, Shin SH, Cho BC, Lee KW, Kim MK, et al. Phase 2 study of dovitinib in patients with metastatic or unresectable adenoid cystic carcinoma. Cancer. 2015; 121:2612–7.
Article9. Ho AL, Dunn L, Sherman EJ, Fury MG, Baxi SS, Chandramohan R, et al. A phase II study of axitinib (AG-013736) in patients with incurable adenoid cystic carcinoma. Ann Oncol. 2016; 27:1902–8.
Article10. Ho AL, Sherman EJ, Baxi SS, Haque S, Ni A, Antonescu CR, et al. Phase II study of regorafenib in progressive, recurrent/metastatic adenoid cystic carcinoma. J Clin Oncol. 2016; 34(15 Suppl):6096.
Article11. Hanna GJ, Ahn MJ, Muzaffar J, Keam B, Bowles DW, Wong DJ, et al. A phase II trial of rivoceranib, an oral vascular endothelial growth factor receptor 2 inhibitor, for recurrent or metastatic adenoid cystic carcinoma. Clin Cancer Res. 2023; 29:4555–63.
Article12. Kang EJ, Ahn MJ, Ock CY, Lee KW, Kwon JH, Yang Y, et al. Randomized phase II study of axitinib versus observation in patients with recurred or metastatic adenoid cystic carcinoma. Clin Cancer Res. 2021; 27:5272–9.
Article13. Jang S, Patel PN, Kimple RJ, McCulloch TM. Clinical outcomes and prognostic factors of adenoid cystic carcinoma of the head and neck. Anticancer Res. 2017; 37:3045–52.14. van der Wal JE, Becking AG, Snow GB, van der Waal I. Distant metastases of adenoid cystic carcinoma of the salivary glands and the value of diagnostic examinations during follow-up. Head Neck. 2002; 24:779–83.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Metastatic Adenoid Cystic Carcinoma of the Lung Diagnosed by Fine Needle Aspiration Biopsy
- A Single-Arm, Prospective, Phase II Study of Cisplatin Plus Weekly Docetaxel as First-Line Therapy in Patients with Metastatic or Recurrent Salivary Gland Cancer
- Cystic Diseases of the Kidney in Chidren
- A case of adenoid basal cell carcinoma in uterine cervix
- A Case of Adenoid Cystic Carcinoma of the Breast Metastatic to the Scalp