Cancer Res Treat.
2024 Oct;56(4):1058-1067. 10.4143/crt.2023.1343.
Metronomic S-1 Adjuvant Chemotherapy Improves Survival in Patients with Locoregionally Advanced Nasopharyngeal Carcinoma
- Affiliations
-
- 1Department of Radiation Oncology, Xiamen Cancer Center, Xiamen Key Laboratory of Radiation Oncology, the First Affiliated Hospital of Xiamen University, School of Medicine, Xiamen University, Xiamen, China
- 2School of Medicine, Sun Yat-sen University, Shenzhen, China
- KMID: 2560240
- DOI: http://doi.org/10.4143/crt.2023.1343
Abstract
- Purpose
This study aimed to investigate the efficacy and safety of using metronomic S-1 adjuvant chemotherapy in locoregionally advanced nasopharyngeal carcinoma (LANPC).
Materials and Methods
We retrospectively collected data on patients diagnosed with LANPC between January 2016 and December 2021. All patients were treated with induction chemotherapy and concurrent chemoradiotherapy with or without metronomic chemotherapy (MC). Toxicities during MC were recorded. The chi-square test, Kaplan-Meier methods, propensity score matching (PSM), and Cox proportional hazards model were used for statistical analyses.
Results
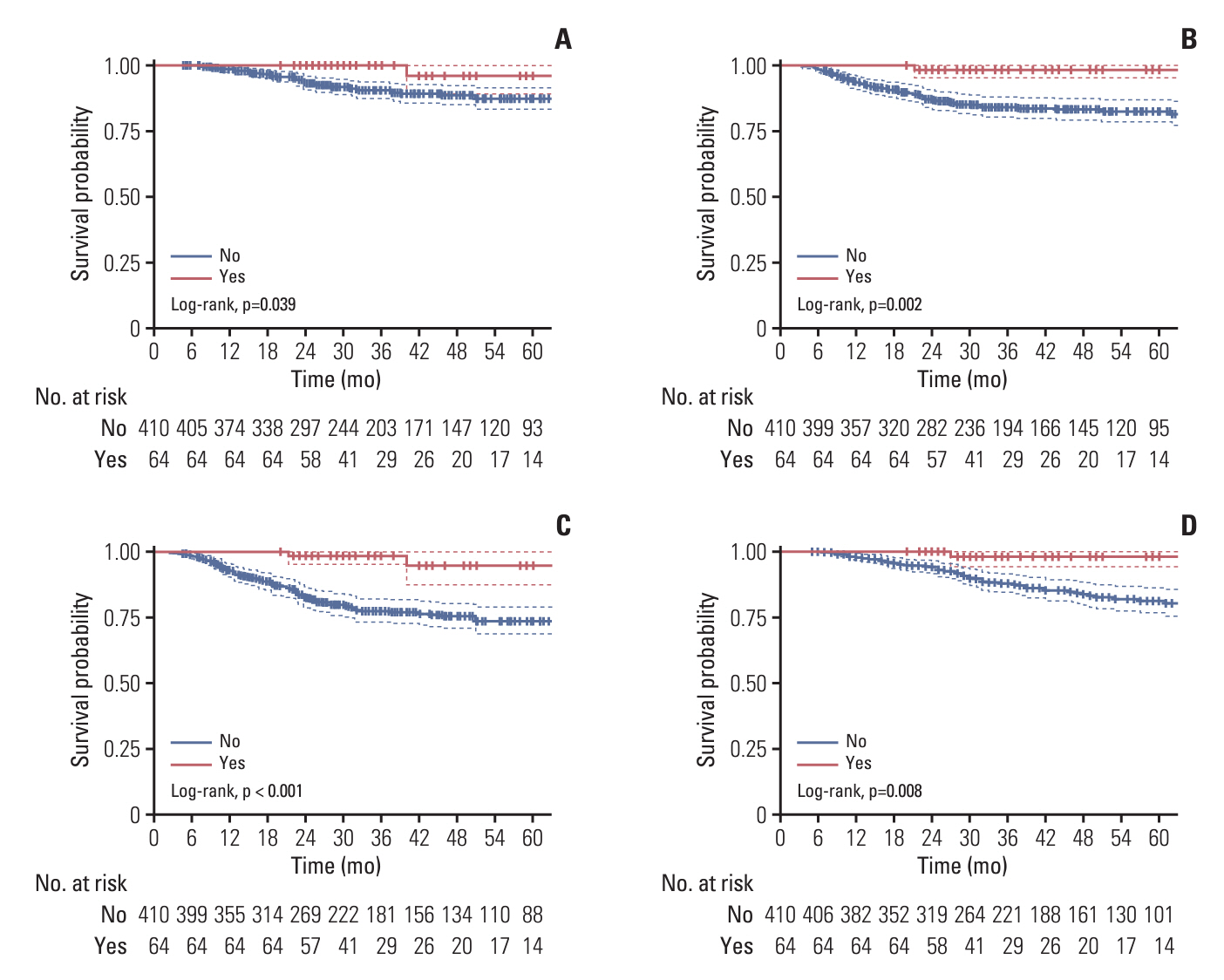
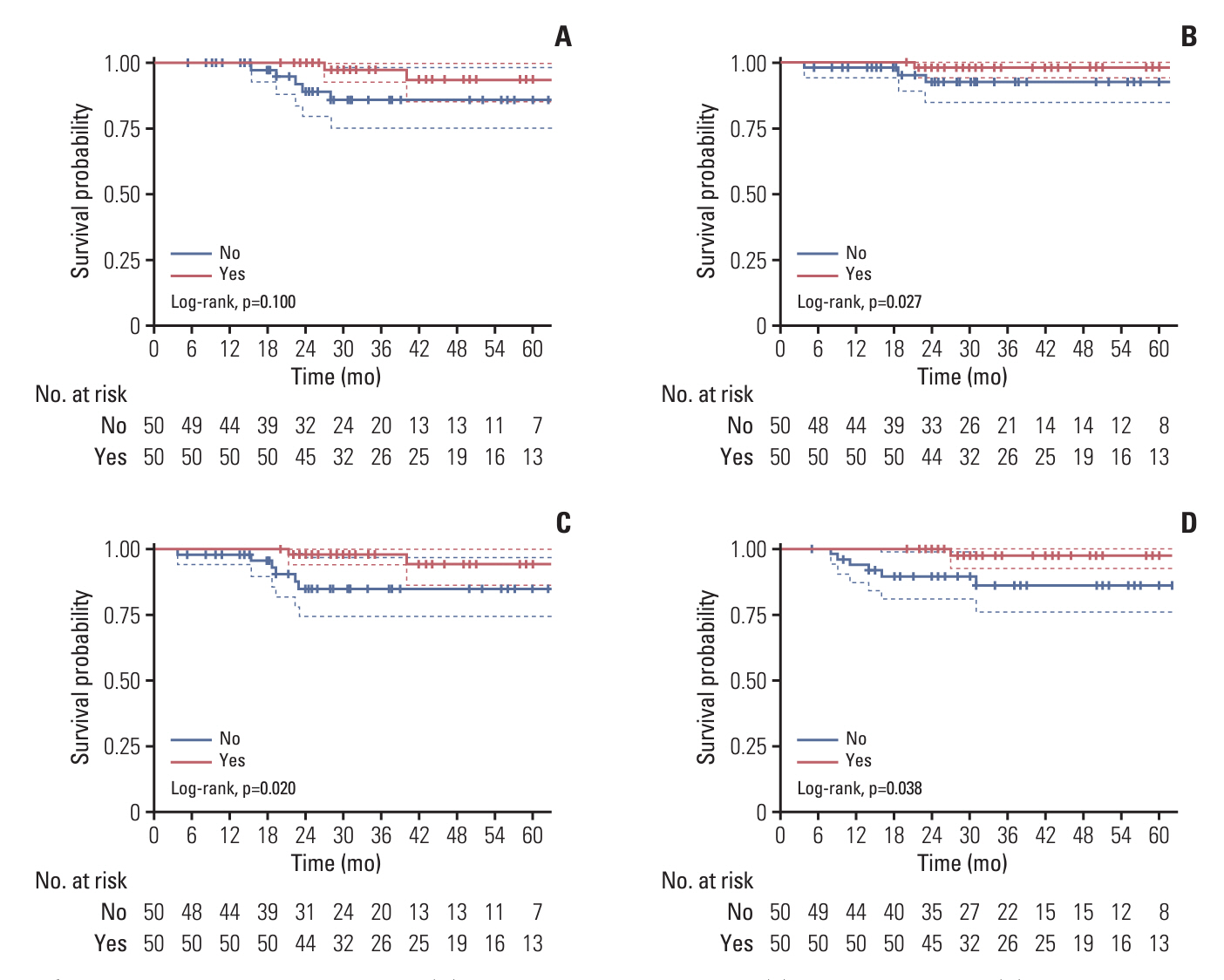
A total of 474 patients were identified, including 64 (13.5%) and 410 (83.5%) patients with or without receiving MC, respectively. Patients who received metronomic S-1 had significantly better 3-year locoregional recurrence-free survival (LRFS) (100% vs. 90.9%, p=0.038), distant metastasis-free survival (DMFS) (98.5% vs. 84.1%, p=0.002), disease-free survival (DFS) (98.4% vs. 77.5%, p < 0.001), and overall survival (OS) (98.0% vs. 87.7%, p=0.008) compared to those without metronomic S-1. The multivariate prognostic analysis revealed that metronomic S-1 was identified as an independent prognostic factor associated with better DMFS (hazard ratio [HR], 0.074; p=0.010), DFS (HR, 0.103; p=0.002) and OS (HR, 0.127; p=0.042), but not in LRFS (p=0.071). Similar results were found using PSM. Common adverse events observed in the metronomic S-1 group included leukopenia, neutropenia, increased total bilirubin, anorexia, rash/desquamation, and hyperpigmentation. All patients with adverse events were grade 1-2.
Conclusion
It is worth conducting a randomized controlled trial to assess the effect of metronomic S-1 on survival outcomes and toxicities of LANPC.
Keyword
Figure
Cited by 2 articles
-
Reply to Commentary on “Metronomic S-1 Adjuvant Chemotherapy Improves Survival in Patients with Locoregionally Advanced Nasopharyngeal Carcinoma”
San-Gang Wu
Cancer Res Treat. 2025;57(1):291-292. doi: 10.4143/crt.2024.1048.Commentary on “Metronomic S-1 Adjuvant Chemotherapy Improves Survival in Patients with Locoregionally Advanced Nasopharyngeal Carcinoma”
Erkan Topkan, Efsun Somay, Nilufer Kılıc Durankus, Ugur Selek
Cancer Res Treat. 2025;57(1):289-290. doi: 10.4143/crt.2024.779.
Reference
-
References
1. Chen YP, Chan AT, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019; 394:64–80.
Article2. Wu CF, Lv JW, Lin L, Mao YP, Deng B, Zheng WH, et al. Development and validation of a web-based calculator to predict individualized conditional risk of site-specific recurrence in nasopharyngeal carcinoma: analysis of 10,058 endemic cases. Cancer Commun (Lond). 2021; 41:37–50.
Article3. Pan JJ, Ng WT, Zong JF, Chan LL, O’Sullivan B, Lin SJ, et al. Proposal for the 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer. 2016; 122:546–58.
Article4. Chen YP, Ismaila N, Chua ML, Colevas AD, Haddad R, Huang SH, et al. Chemotherapy in combination with radiotherapy for definitive-intent treatment of stage II-IVA nasopharyngeal carcinoma: CSCO and ASCO guideline. J Clin Oncol. 2021; 39:840–59.
Article5. Tang LL, Chen YP, Chen CB, Chen MY, Chen NY, Chen XZ, et al. The Chinese Society of Clinical Oncology (CSCO) clinical guidelines for the diagnosis and treatment of nasopharyngeal carcinoma. Cancer Commun (Lond). 2021; 41:1195–227.
Article6. Chen YP, Tang LL, Yang Q, Poh SS, Hui EP, Chan AT, et al. Induction chemotherapy plus concurrent chemoradiotherapy in endemic nasopharyngeal carcinoma: individual patient data pooled analysis of four randomized trials. Clin Cancer Res. 2018; 24:1824–33.
Article7. Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD, Yang KY, et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med. 2019; 381:1124–35.8. Lv J, Chen Y, Zhou G, Qi Z, Tan KR, Wang H, et al. Liquid biopsy tracking during sequential chemo-radiotherapy identifies distinct prognostic phenotypes in nasopharyngeal carcinoma. Nat Commun. 2019; 10:3941.
Article9. Ko JM, Vardhanabhuti V, Ng WT, Lam KO, Ngan RK, Kwong DL, et al. Clinical utility of serial analysis of circulating tumour cells for detection of minimal residual disease of metastatic nasopharyngeal carcinoma. Br J Cancer. 2020; 123:114–25.
Article10. Chen L, Hu CS, Chen XZ, Hu GQ, Cheng ZB, Sun Y, et al. Adjuvant chemotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase 3 multicentre randomised controlled trial. Eur J Cancer. 2017; 75:150–8.
Article11. Chan AT, Hui EP, Ngan RK, Tung SY, Cheng AC, Ng WT, et al. Analysis of plasma Epstein-Barr virus DNA in nasopharyngeal cancer after chemoradiation to identify high-risk patients for adjuvant chemotherapy: a randomized controlled trial. J Clin Oncol. 2018; 36:3091–100.
Article12. Wang X, Wang SS, Huang H, Cai L, Zhao L, Peng RJ, et al. Effect of capecitabine maintenance therapy using lower dosage and higher frequency vs observation on disease-free survival among patients with early-stage triple-negative breast cancer who had received standard treatment: the SYSUCC-001 randomized clinical trial. JAMA. 2021; 325:50–8.
Article13. Woo IS, Jung YH. Metronomic chemotherapy in metastatic colorectal cancer. Cancer Lett. 2017; 400:319–24.
Article14. Xu K, Liu T, Zhang J, Zhou Y, Yang F, Ren T. The efficacy and toxicity of metronomic oral vinorelbine monotherapy in patients with non-small cell lung cancer: a meta-analysis. Int J Clin Oncol. 2020; 25:1624–34.
Article15. Chen YP, Liu X, Zhou Q, Yang KY, Jin F, Zhu XD, et al. Metronomic capecitabine as adjuvant therapy in locoregionally advanced nasopharyngeal carcinoma: a multicentre, open-label, parallel-group, randomised, controlled, phase 3 trial. Lancet. 2021; 398:303–13.
Article16. Lu Y, Huang H, Yang H, Hu X, Liu M, Huang C, et al. Maintenance therapy improves the survival outcomes of patients with metastatic nasopharyngeal carcinoma responding to first-line chemotherapy: a multicentre, randomized controlled clinical study. J Cancer Res Clin Oncol. 2023; 149:4327–38.
Article17. Zong J, Xu H, Chen B, Guo Q, Xu Y, Chen C, et al. Maintenance chemotherapy using S-1 following definitive chemoradiotherapy in patients with N3 nasopharyngeal carcinoma. Radiat Oncol. 2019; 14:182.
Article18. Zhang S, Zhou L, Huang X, Lin S. A retrospective study of concurrent chemoradiotherapy plus S-1 adjuvant chemotherapy on curative effect for treatment of patients with N3 stage nasopharyngeal carcinoma. Cancer Manag Res. 2018; 10:1705–11.
Article19. Tao HY, He F, Shi QY, Liu R, Wang ZL, Du KP, et al. Efficacy of adjuvant chemotherapy/maintenance chemotherapy after induction chemotherapy and concurrent chemoradiotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: experiences of two centers. Cancer Med. 2023; 12:6811–24.
Article20. Zong J, Liu Y, Liang Q, Xu H, Chen B, Guo Q, et al. Administration of oral maintenance chemotherapy for 1 year following definitive chemoradiotherapy may improve the survival of patients with stage N3 nasopharyngeal carcinoma. Oral Oncol. 2021; 118:105313.21. Choi S, Min JS, Jeong SH, Yoo MW, Son YG, Oh SJ, et al. Long-term survival outcomes of elderly patients treated with S-1 or capecitabine plus oxaliplatin for stage II or III gastric cancer: a multicenter cohort study. J Gastric Cancer. 2022; 22:67–77.
Article22. Li J, You J, Si W, Zhu Y, Chen Y, Yang B, et al. Docetaxel/S-1 versus docetaxel/capecitabine as first-line treatment for advanced breast cancer: a retrospective study. Medicine (Baltimore). 2015; 94:e1340.23. He AB, Peng XL, Song J, Zhang JX, Dong WG, Luo RF, et al. Efficacy of S-1 vs capecitabine for the treatment of gastric cancer: a meta-analysis. World J Gastroenterol. 2015; 21:4358–64.
Article24. Zheng H, Zhou P, Wang J, Yu YF, Zhou R, Lin Q, et al. Prognostic effect of residual plasma Epstein-Barr viral DNA after induction chemotherapy for locoregionally advanced nasopharyngeal carcinoma. Cancer Med. 2023; 12:14979–87.25. Huang CL, Wang GY, Lou JH, Chen L, Li QJ, Li KP, et al. Oral chemotherapy versus observation alone in nasopharyngeal carcinoma patients with persistently detected circulating cell-free Epstein-Barr virus DNA during follow-up. Radiother Oncol. 2024; 190:110032.
Article26. Twu CW, Wang WY, Chen CC, Liang KL, Jiang RS, Wu CT, et al. Metronomic adjuvant chemotherapy improves treatment outcome in nasopharyngeal carcinoma patients with postradiation persistently detectable plasma Epstein-Barr virus deoxyribonucleic acid. Int J Radiat Oncol Biol Phys. 2014; 89:21–9.
Article27. She L, Tian K, Han J, Zuo W, Wang Z, Zhang N. Cost-effectiveness analysis of metronomic capecitabine as adjuvant chemotherapy in locoregionally advanced nasopharyngeal carcinoma. Front Oncol. 2022; 12:904372.
Article28. Caudell JJ, Gillison ML, Maghami E, Spencer S, Pfister DG, Adkins D, et al. NCCN Guidelines(R) insights: head and neck cancers, version 1.2022. J Natl Compr Canc Netw. 2022; 20:224–34.29. Tsukahara K, Kubota A, Hasegawa Y, Takemura H, Terada T, Taguchi T, et al. Randomized phase III trial of adjuvant chemotherapy with S-1 after curative treatment in patients with squamous-cell carcinoma of the head and neck (ACTS-HNC). PLoS One. 2015; 10:e0116965.
Article30. Kitani Y, Kubota A, Furukawa M, Hori Y, Nakayama Y, Nonaka T, et al. Impact of combined modality treatment with radiotherapy and S-1 on T2N0 laryngeal cancer: possible improvement in survival through the prevention of second primary cancer and distant metastasis. Oral Oncol. 2017; 71:54–9.
Article31. Furusaka T, Tanaka A, Matsuda H, Ikeda M. Consecutive daily low-dose S-1 adjuvant chemotherapy after radical treatment for squamous cell carcinoma in head and neck cancer. Acta Otolaryngol. 2011; 131:1099–103.
Article32. Andre N, Tsai K, Carre M, Pasquier E. Metronomic chemotherapy: direct targeting of cancer cells after all? Trends Cancer. 2017; 3:319–25.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Commentary on “Metronomic S-1 Adjuvant Chemotherapy Improves Survival in Patients with Locoregionally Advanced Nasopharyngeal Carcinoma”
- Reply to Commentary on “Metronomic S-1 Adjuvant Chemotherapy Improves Survival in Patients with Locoregionally Advanced Nasopharyngeal Carcinoma”
- Less is more: role of additional chemotherapy to concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal cancer management
- Locoregionally advanced nasopharyngeal carcinoma treated with intensity-modulated radiotherapy plus concurrent weekly cisplatin with or without neoadjuvant chemotherapy
- Can metronomic chemotherapy be an alternative to sorafenib in advanced hepatocellular carcinoma?