J Rheum Dis.
2024 Oct;31(4):230-243. 10.4078/jrd.2024.0043.
Clinical characteristics of chronic sclerosing sialadenitis as a distinctive entity from primary Sjögren’s syndrome
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2559892
- DOI: http://doi.org/10.4078/jrd.2024.0043
Abstract
Objective
This study aimed to elucidate the clinical and laboratory differences between chronic sclerosing sialadenitis (CSS) and primary Sjögren’s syndrome (pSS), highlighting CSS as a distinct pathological entity within the spectrum of salivary gland pathology.
Methods
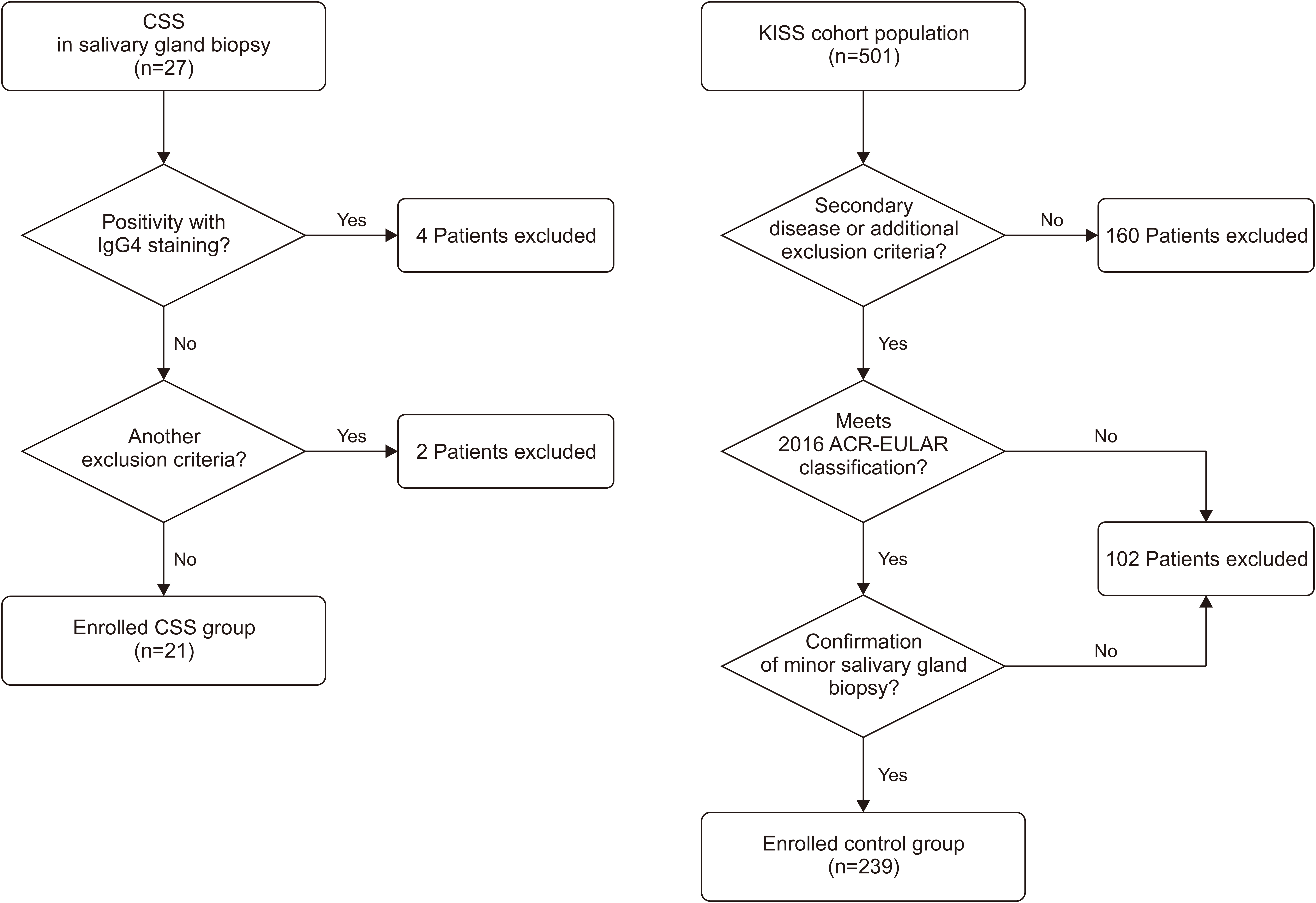
This retrospective, single-center study was conducted at Seoul St. Mary’s Hospital between January 2000 and December 2022. Patients diagnosed with CSS via salivary gland biopsy were included, and those with IgG4-related disease (IgG4-RD) or other confounding factors were excluded. Clinical and laboratory CSS profiles were compared with those of a control group of patients with typical pSS from the Korean Initiative of Primary Sjögren’s Syndrome (KISS) prospective cohort study. Twenty-one with CSS and 501 patients with pSS from Seoul St. Mary’s Hospital were retrospectively analyzed.
Results
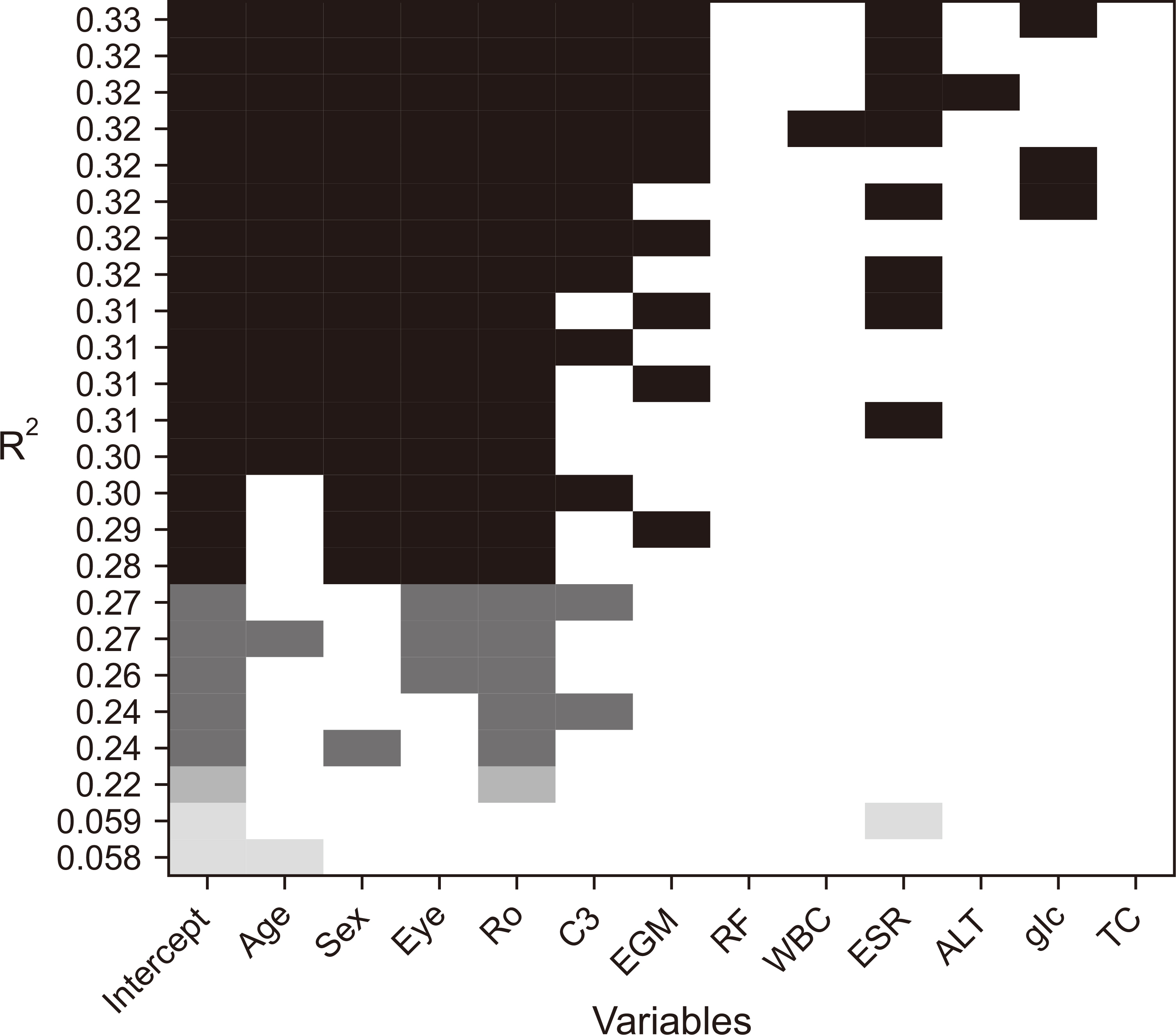
Patients with CSS were older at diagnosis, had a lower prevalence of ocular symptoms, and exhibited distinct immunological markers compared to those with pSS. Logistic regression analysis revealed that anti-Ro antibody positivity, elevated erythrocyte sedimentation rate levels, low serum complement 3 levels, and accompanying dry eye symptoms were factors distinguishing pSS from CSS.
Conclusion
Even after excluding IgG4-RD, CSS was significantly different from pSS in terms of clinical and laboratory findings. Recognition of these differences is crucial for the accurate diagnosis and management of CSS, underscoring its status as a distinct pathological entity among salivary gland pathologies.
Keyword
Figure
Reference
-
1. Negrini S, Emmi G, Greco M, Borro M, Sardanelli F, Murdaca G, et al. 2022; Sjögren's syndrome: a systemic autoimmune disease. Clin Exp Med. 22:9–25. DOI: 10.1007/s10238-021-00728-6. PMID: 34100160. PMCID: PMC8863725.2. Oxholm P, Asmussen K. 1995; Classification of disease manifestations in primary Sjögren's syndrome: present status and a new proposal. Clin Rheumatol. 14 Suppl 1:3–7. DOI: 10.1007/BF03342630.3. Mihai A, Caruntu C, Jurcut C, Blajut FC, Casian M, Opris-Belinski D, et al. 2023; The spectrum of extraglandular manifestations in primary Sjögren's syndrome. J Pers Med. 13:961. DOI: 10.3390/jpm13060961. PMID: 37373950. PMCID: PMC10305413.4. Trevisani VFM, Pugliesi A, Pasoto SG, Lopes MLL, Guedes LKN, Miyamoto ST, et al. 2022; Recommendations for evaluation and diagnosis of extra-glandular manifestations of primary sjogren syndrome: results of an epidemiologic systematic review/meta-analysis and a consensus guideline from the Brazilian Society of Rheumatology (articular, pulmonary and renal). Adv Rheumatol. 62:18. DOI: 10.1186/s42358-022-00248-1. PMID: 35650656.5. Kabasakal Y, Kitapçıoğlu G, Karabulut G, Tezcan M, Balkarlı A, Aksoy A, et al. 2017; Criteria sets for primary Sjogren's syndrome are not adequate for those presenting with extraglandular organ involvements as their dominant clinical features. Rheumatol Int. 37:675–84. DOI: 10.1007/s00296-017-3691-8.6. Goules AV, Tzioufas AG, Moutsopoulos HM. 2014; Classification criteria of Sjögren's syndrome. J Autoimmun. 48-49:42–5. DOI: 10.1016/j.jaut.2014.01.013. PMID: 24456935.7. Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. 2002; Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 61:554–8. DOI: 10.1136/ard.61.6.554. PMID: 12006334. PMCID: PMC1754137.8. Shiboski SC, Shiboski CH, Criswell L, Baer A, Challacombe S, Lanfranchi H, et al. 2012; American College of Rheumatology classification criteria for Sjögren's syndrome: a data-driven, expert consensus approach in the Sjögren's International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken). 64:475–87. DOI: 10.1002/acr.21591. PMID: 22563590. PMCID: PMC3349440.9. Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, et al. 2017; 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren's syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. 69:35–45. DOI: 10.1002/art.39859. PMID: 27785888. PMCID: PMC5650478.10. Tuchocka-Piotrowska A, Puszczewicz M, Kołczewska A, Majewski D. 2006; [Graft-versus-host disease as the cause of symptoms mimicking Sjögren's syndrome]. Ann Acad Med Stetin. 52 Suppl 2:89–93. Polish. PMID: 17474179.11. Otani Y, Shimura T, Nogaki T, Ikenoya Y, Oyake K, Imaizumi N, et al. 2022; Differentiation between IgG4-related Mikulicz disease and Sjögren's syndrome: a review case report and literature review. Medicine (Baltimore). 101:e32617. DOI: 10.1097/MD.0000000000032617. PMID: 36596084. PMCID: PMC9803436.12. Fragoulis GE, Zampeli E, Moutsopoulos HM. 2017; IgG4-related sialadenitis and Sjögren's syndrome. Oral Dis. 23:152–6. DOI: 10.1111/odi.12526. PMID: 27318181.13. Baer AN, Walitt B. 2018; Update on Sjögren syndrome and other causes of sicca in older adults. Rheum Dis Clin North Am. 44:419–36. DOI: 10.1016/j.rdc.2018.03.002. PMID: 30001784. PMCID: PMC6245643.14. Markowski J, Gierek T, Witkowska M, Paluch J, Swiderek M, Zielińska-Pająk E, et al. 2011; [Küttner's tumor (chronic sclerosing sialadenitis) - a rare cause of submandibular gland enlargement]. Otolaryngol Pol. 65:289–92. Polish. DOI: 10.1016/S0030-6657(11)70693-X. PMID: 22000148.15. Weitz-Tuoretmaa A, Laranne J, Paloneva T, Michelaki K. 2013; [Chronic sclerosing sialadenitis - Küttner tumor]. Duodecim. 129:2280–3. Finnish. PMID: 24340679.16. Williams HK, Connor R, Edmondson H. 2000; Chronic sclerosing sialadenitis of the submandibular and parotid glands: a report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 89:720–3. DOI: 10.1067/moe.2000.102515. PMID: 10846127.17. Bagnoli S. 1957; [Unusual histopathological variant of so-called Kuttner's inflammatory tumor of the sublingual region: giant-cell sclero-atrophic chronic sialadenitis]. Arch De Vecchi Anat Patol. 26:655–68. Italian. PMID: 13509871.18. Gentili M. 1966; [Considerations on a rare disease of the salivary glands. Kuttner's inflammatory "pseudo-tumor"]. Ann Stomatol (Roma). 15:95–108. Italian.19. Seifert G, Donath K. 1977; [On the pathogenesis of the Küttner tumor of the submandibular gland -- analysis of 349 cases with chronic sialadenitis of the submandibular (author's transl)]. HNO. 25:81–92. German. PMID: 856776.20. Cheuk W, Chan JK. 2007; Advances in salivary gland pathology. Histopathology. 51:1–20. DOI: 10.1111/j.1365-2559.2007.02719.x. PMID: 17539914.21. Jham BC, Freire ARS, da Silveira-Júnior JB, de Aguiar MCF, Mesquita RA. 2006; Kuttner tumor involving minor salivary glands in a patient undergoing radiotherapy in the head and neck. Oral Oncol Extra. 42:181–3. DOI: 10.1016/j.ooe.2005.11.004.22. Blanco M, Mesko T, Cura M, Cabello-Inchausti B. 2003; Chronic sclerosing sialadenitis (Kuttner's tumor): unusual presentation with bilateral involvement of major and minor salivary glands. Ann Diagn Pathol. 7:25–30. DOI: 10.1053/adpa.2003.50004. PMID: 12616471.23. Paul R, Shekar K, Singh M. 2010; Kuttner tumour: an unusual cause of enlargement of a minor salivary gland in the lip. Br J Oral Maxillofac Surg. 48:152–3. DOI: 10.1016/j.bjoms.2009.07.029. PMID: 19926183.24. De Cocker LJ, D'Arco F, De Beule T, Tousseyn T, Blockmans D, Hermans R. 2014; IgG4-related systemic disease affecting the parotid and submandibular glands: magnetic resonance imaging features of IgG4-related chronic sclerosing sialadenitis and concomitant lymphadenitis. Clin Imaging. 38:195–8. DOI: 10.1016/j.clinimag.2013.11.002. PMID: 24332556.25. Kamiński B, Błochowiak K. 2020; Mikulicz's disease and Küttner's tumor as manifestations of IgG4-related diseases: a review of the literature. Reumatologia. 58:243–50. DOI: 10.5114/reum.2020.98437. PMID: 32921832. PMCID: PMC7477471.26. Wei TW, Lien CF, Hsu TY, He HL. 2015; Chronic sclerosing sialadenitis of the submandibular gland: an entity of IgG4-related sclerosing disease. Int J Clin Exp Pathol. 8:8628–31. PMID: 26339446. PMCID: PMC4555774.27. Kitagawa S, Zen Y, Harada K, Sasaki M, Sato Y, Minato H, et al. 2005; Abundant IgG4-positive plasma cell infiltration characterizes chronic sclerosing sialadenitis (Küttner's tumor). Am J Surg Pathol. 29:783–91. DOI: 10.1097/01.pas.0000164031.59940.fc. PMID: 15897744.28. Koh JH, Lee J, Chung SH, Kwok SK, Park SH. 2019; Phenotypic features and predictors of the clinical severity of keratoconjunctivitis sicca and salivary gland dysfunction in patients with Sjögren's syndrome: a longitudinal analysis of the Korean Initiative of primary Sjögren's Syndrome (KISS) cohort. Scand J Rheumatol. 48:198–206. DOI: 10.1080/03009742.2018.1504982. PMID: 30475093.29. Koh JH, Park Y, Lee J, Park SH, Kwok SK. 2021; Hypergammaglobulinaemia predicts glandular and extra-glandular damage in primary Sjögren's syndrome: results from the KISS cohort study. Clin Exp Rheumatol. 39(6 Suppl 133):114–22. DOI: 10.55563/clinexprheumatol/volsh1. PMID: 34796856.30. Soares AB, Faria PR, Magna LA, Correa ME, de Sousa CA, Almeida OP, et al. 2005; Chronic GVHD in minor salivary glands and oral mucosa: histopathological and immunohistochemical evaluation of 25 patients. J Oral Pathol Med. 34:368–73. DOI: 10.1111/j.1600-0714.2005.00322.x. PMID: 15946186.31. Nagler R, Marmary Y, Krausz Y, Chisin R, Markitziu A, Nagler A. 1996; Major salivary gland dysfunction in human acute and chronic graft-versus-host disease (GVHD). Bone Marrow Transplant. 17:219–24. PMID: 8640170.32. Ferreiro MC, Prieto MH, Rodríguez SB, Vázquez RL, Iglesias AC, Dios PD. 2002; Whole stimulated salivary flow in patients with chronic hepatitis C virus infection. J Oral Pathol Med. 31:117–20. DOI: 10.1046/j.0904-2512.2001.00185.x. PMID: 11896834.33. Myssiorek D, Alvi A, Bhuiya T. 1992; Primary salivary gland amyloidosis causing sicca syndrome. Ann Otol Rhinol Laryngol. 101:487–90. DOI: 10.1177/000348949210100607. PMID: 1376976.34. de Oliveira NC, de Oliveira TC, Klamas VC, Ventura MA, Kamei AA, Naka JY, et al. 2020; Salivary flow, amylase, and total protein in hospitalized patients with HIV infection /AIDS complications. Afr Health Sci. 20:597–604. DOI: 10.4314/ahs.v20i2.7. PMID: 33163020. PMCID: PMC7609095.35. Seror R, Bowman SJ, Brito-Zeron P, Theander E, Bootsma H, Tzioufas A, et al. 2015; EULAR Sjögren's syndrome disease activity index (ESSDAI): a user guide. RMD Open. 1:e000022. DOI: 10.1136/rmdopen-2014-000022. PMCID: PMC4613159.36. Seror R, Ravaud P, Mariette X, Bootsma H, Theander E, Hansen A, et al. 2011; EULAR Sjogren's Syndrome Patient Reported Index (ESSPRI): development of a consensus patient index for primary Sjogren's syndrome. Ann Rheum Dis. 70:968–72. DOI: 10.1136/ard.2010.143743. PMID: 21345815.37. Ramos-Casals M, Brito-Zerón P, Seror R, Bootsma H, Bowman SJ, Dörner T, et al. 2015; Characterization of systemic disease in primary Sjögren's syndrome: EULAR-SS Task Force recommendations for articular, cutaneous, pulmonary and renal involvements. Rheumatology (Oxford). 54:2230–8. Erratum in: Rheumatology (Oxford) 2017;56:1245. DOI: 10.1093/rheumatology/kev200. PMID: 28379527. PMCID: PMC7191868.38. Frisell T, Bower H, Morin M, Baecklund E, Di Giuseppe D, Delcoigne B, et al. 2023; Safety of biological and targeted synthetic disease-modifying antirheumatic drugs for rheumatoid arthritis as used in clinical practice: results from the ARTIS programme. Ann Rheum Dis. 82:601–10. DOI: 10.1136/ard-2022-223762. PMID: 36787994. PMCID: PMC10176333.39. Dreher M, Witte T, Hoeper K, Assmann G, Proft F, Poddubnyy D, et al. 2024; Rheuma-VOR study: optimising healthcare of rheumatic diseases by multiprofessional coordinating centres. Ann Rheum Dis. 83:184–93. DOI: 10.1136/ard-2023-224205. PMID: 37890976. PMCID: PMC10850684.40. Gönen M. 2004; Sample size and power for McNemar's test with clustered data. Stat Med. 23:2283–94. DOI: 10.1002/sim.1768. PMID: 15236431.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fine Needle Aspiration Cytology of Chronic Sclerosing Sialadenitis with Mucinous Metaplasia in Parotid Gland: A Case Report
- Various Sonographic Findings of Kuttner Tumors of the Bilateral Submandibular Gland and Right Parotid Gland
- A Case of Sjogren's Syndrome in a Patient with Hashimoto's Thyroiditis
- Two Cases of Acute Cerebral Infarction Associated with Primary Sjogren's Syndrome
- Primary Sjogren's Syndrome Associated with Membranous Glomerulonephritis