Ann Clin Microbiol.
2024 Sep;27(3):205-214. 10.5145/ACM.2024.27.3.4.
Verification of the Mycoplasma IST3 for urogenital mycoplasma culture in comparison to the Mycoplasma IST2
- Affiliations
-
- 1Department of Laboratory Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2559814
- DOI: http://doi.org/10.5145/ACM.2024.27.3.4
Abstract
- Background
Unlike the Mycoplasma IST2 kit (bioMérieux), the Mycoplasma IST3 kit has been updated to comply with the standardized antimicrobial susceptibility test (AST) method for Ureaplasma spp. (Up) and Mycoplasma hominis (Mh). We aimed to verify the use of the Mycoplasma IST3 kit for genital mycoplasma cultures.
Methods
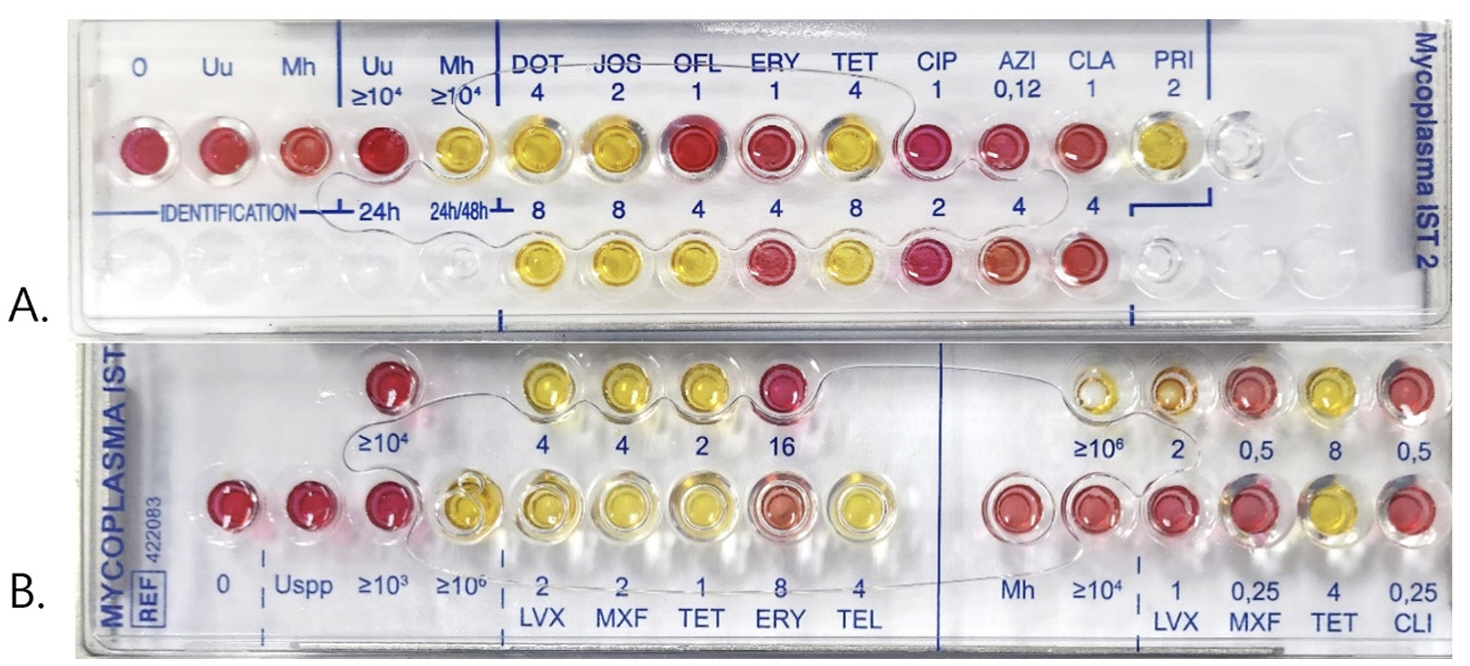
From September 2023 to January 2024, the R1 medium remaining after inoculation with IST2 was refrigerated until the next day. For IST2-positive samples, 300 μL of residual R1 medium was inoculated into the IST3. Species identification, enumeration, and AST results obtained using IST3 were compared with those obtained using IST2.
Results
A total of 48 IST2-positive samples were inoculated into IST3, including 35, 1, and 12 Up-only, Mh-only, and both Up- and Mh-positive samples, respectively. Among Up-only samples, 2.8%, 91.4%, and 100.0% were susceptible to ciprofloxacin, tetracycline, and erythromycin, respectively. With IST3, 45 (93.8%) samples grew genital mycoplasmas; 42 (89.4%) of the 47 Up-positive samples and 6 (46.2%) of the 13 Mh-positive samples showed growth of the same organisms. All seven samples that failed to grow Mh were from mixed cultures, of which four Mh concentrations of < 104 /mL. Up was susceptible to levofloxacin, tetracycline, and erythromycin at the rates of 64.3 %, 88.1 %, and 95.2 %, respectively.
Conclusion
IST3 showed good performance in detecting genital Mycoplasma except for its tendency to not detect Mh of low concentrations in mixed cultures. IST3 is preferable to IST2 because it can accurately screen for erythromycin resistance in Up and reduce falseresistances for fluoroquinolone.
Keyword
Figure
Reference
-
1. Waites KB and Bébéar C. Mycoplasma and Ureaplasma. In: Caroll KC, et al. eds. Manual of Clinical Microbiology. 13th ed. Washington DC; American Society of Microbiology: 2023.2. Chang YS, Kim SG, Kim BI, Park WS, Yoon BH, Kim EC, et al. Genital mycoplasma in the newborn infants: colonization, prevalence and clinical significance. J Korean Pediatr Soc 1996;30:1084-94.3. Koh E, Kim S, Kim IS, Maeng KY, Lee SA. Antimicrobial susceptibilities of Ureaplasma urealyticum and Mycoplasma hominis in pregnant women. Korean J Clin Microbiol 2009;12:159-62.4. Chung HK, Park SY, Park MH, Kim YJ, Chun SH, Cho SJ, et al. Association of genital mycoplasmas infection in women who had preterm delivery and outcomes in premature infants. Korean J Obstet Gynecol 2012;55:158-65.5. D'Inzeo T, Angelis G, Fiori B, Menchinelli G, Liotti FM, Morandotti GM. Comparison of Mycoplasma IES, Mycofast Revolution and Mycoplasma IST2 to detect genital mycoplasmas in clinical samples. J Infect Dev Ctries 2017;11:98-101.6. Park HR, Kim YH, Lee HJ, Oh JS, Kim HJ. Usefulness of the Mycofast test (MYCOFAST® Evolution 2) for the diagnosis of nongonococcal genitourinary infections. Korean J Urol 2006;47:1117-23.7. Wen X, Nobakht MS, Yang Y, Kouhsari E, Hajilari S, Shakourzadeh MZ, et al. Tetracyclines resistance in Mycoplasma and Ureaplasma urogenital isolates derived from human: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob 2023;22:83.8. Song J, Wu X, Kong Y, Jin H, Yang T, Xie X, et al. Prevalence and antibiotics resistance of Ureaplasma species and Mycoplasma hominis in Hangzhou, China, from 2013 to 2019. Front Microbiol 2022;13:982429.9. Mycoplasma IST2. Diagnosis of urogenital mycoplasma (culture, identification, enumeration, and susceptibility testing). Package insert. bioMérieux REF 42505.10. Mycoplasma IST3. Diagnosis of urogenital mycoplasma (culture, identification, enumeration, and susceptibility testing). Package insert. bioMérieux REF 422083.11. Clinical and Laboratory Standards Institute. Methods for antimicrobial susceptibility testing for human mycoplasmas—first edition: M43-A. Wayne; CLSI: 2011.12. Boostrom I, Bala Y, Vasic JM, Gluvakov J, Chanard E, Barratt AH. Evaluation of the Mycoplasma IST3 urogenital mycoplasma assay in an international multicentre trial. J Antimicrob Chemother 2021;76:3175-82.13. Choe HS, Lee DS, Lee SJ, Hong SH, Park DC, Lee MK, et al. Performance of Anyplex™ II multiplex real-time PCR for the diagnosis of seven sexually transmitted infections: comparison with currently available methods. Int J Infect Dis 2013;17:e1134-40.14. Min SK, Kim SK, Kim YS, Cho IC, Lee GI. Evaluation of clinical sample for Accupower UU Real-Time PCR kit. Korean J Urogenit Tract Infect Inflamm 2014;9:99-103.15. Chang J, Yu JK, Song C, Park IY, Park YJ. Prevalence and antimicrobial susceptibility of genital Mycoplasmataceae in Korean women: correlation between phenotypic test and resistance genes. Ann Clin Microbiol 2016;19:13-9.16. Kweon OJ, Lim YK, Oh SM, Kim TH, Choe HS, Lee SJ, et al. Prevalence and antimicrobial susceptibility of Mycoplasma hominis, Ureaplasma urealyticum and Ureaplasma parvum in individuals with or without symptoms of genitourinary infections. Lab Med Online 2016;6:7987.17. Lee JY and Yang JS. Prevalence and antimicrobial susceptibility of Mycoplasma hominis and Ureaplasma species in nonpregnant female patients in South Korea indicate an increasing trend of pristinamycin-resistant isolates. Antimicrob Agents Chemother 2020;64:e01065-20.18. Chung HY, Chung JW, Chun SH, Sung H, Kim MN, Kim KS. A case of erythromycinresistant Ureaplasma urealyticum meningitis in a premature infant. Korean J Lab Med 2007;27:46-9.