J Korean Med Sci.
2024 Oct;39(38):e259. 10.3346/jkms.2024.39.e259.
Examining the Relationship Between Polystyrene Microplastics and Male Fertility: Insights From an In Vivo Study and In Vitro Sertoli Cell Culture
- Affiliations
-
- 1Department of Urology, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea
- 2Department of Nephrology, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea
- 3Department of Radiology, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea
- 4Division of Rhinology, Department of Otorhinolaryngology-Head and Neck Surgery, Korea University College of Medicine, Ansan, Korea
- KMID: 2559784
- DOI: http://doi.org/10.3346/jkms.2024.39.e259
Abstract
- Background
While polystyrene microplastics (PS-MPs) are emerging as potentially significant health threats, linked to cancer and reproductive dysfunction, their precise effects on human health remain largely unknown. We aimed to investigate the underlying mechanisms promoting microplastic-induced damage in the reproductive system.
Methods
Thirty C57BL/6 male mice were randomly allocated into six equal-sized groups. Mice were exposed to fluorescent PS-MPs (5 µm, < 18%, green) at a dose of 1 and 3 mg/dL via oral gavage for 28 and 56 days, respectively (control, 0 mg/dL). The presence of antibodies and inflammatory and oxidative stress markers were evaluated using western blotting. Sperm analysis was also performed. Mouse testis Sertoli TM4 cells were divided into two groups: control (medium only) and PS-MPs (medium containing, 1,000 μg/mL) groups and cultured in vitro for 1, 24, 48, or 72 hours. The cells were cultured in a Ham’s F12: Dulbecco's Modified Eagle Medium medium with 0.25% fetal bovine serum at 37°C with humidified atmosphere of 5% carbon dioxide in the air. Protein analyses for interleukin (IL)-6, IL-10, NADPH-oxidase (NOX)-2, NOX-4, hypoxia-inducible transcription factor (HIF)-2α, monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor (TNF)-α, and transforming growth factor (TGF)-β were performed using western blotting.
Results
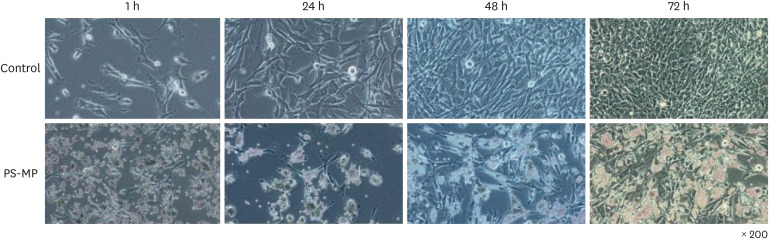
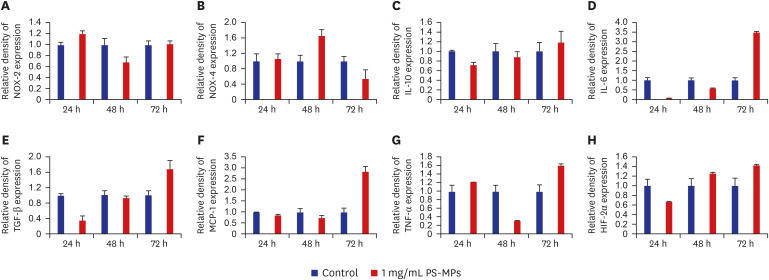
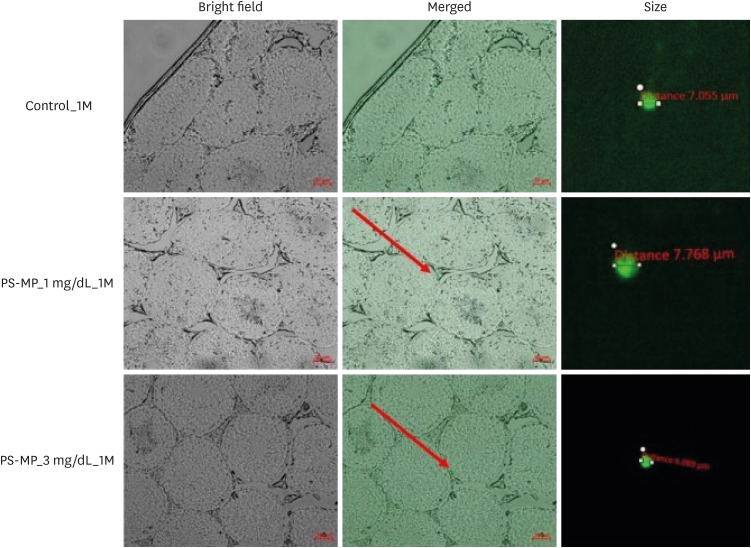
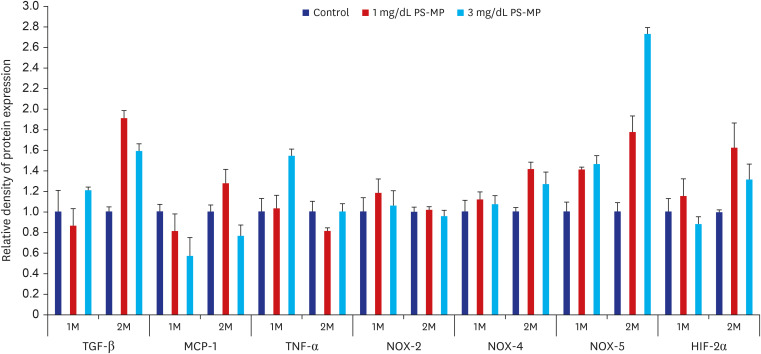
The testes were evaluated after 28 and 56 days of exposure. Varying sizes of PS-MPs were detected in the testes (ranging from 5.870 to 7.768 µm). Significant differences in sperm concentration, motility, and the proportion of normal sperm were observed between the two groups. An increase in TGF-β, HIF-2α, and NOX-4 levels was observed using western blot analysis. However, no dose-dependent correlations were observed between the two groups. In vitro evaluation of the PS-MPs group displayed PS-MP penetration of the lumen of Sertoli cells after 1 hour. Further PS-MP aggregation within Sertoli cells was observed at 24, 48, and 72 hours. A significant increase in inflammatory protein expressions (IL-10, TGF-β, MCP-1, IL-6, TNF-α, and HIF-2α) was observed through western blotting, although oxidative agents did not show a significant increase.
Conclusion
PS-MPs induced reproductive dysfunction in male mice provide new insights into PS-MPs-associated toxicity in mammals.
Keyword
Figure
Reference
-
1. Hartmann NB, Hüffer T, Thompson RC, Hassellöv M, Verschoor A, Daugaard AE, et al. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ Sci Technol. 2019; 53(3):1039–1047. PMID: 30608663.2. de Souza Machado AA, Kloas W, Zarfl C, Hempel S, Rillig MC. Microplastics as an emerging threat to terrestrial ecosystems. Glob Change Biol. 2018; 24(4):1405–1416.3. Prata JC. Airborne microplastics: consequences to human health? Environ Pollut. 2018; 234:115–126. PMID: 29172041.4. Kannan K, Vimalkumar K. A review of human exposure to microplastics and insights into microplastics as obesogens. Front Endocrinol (Lausanne). 2021; 12:724989. PMID: 34484127.5. Mitrano DM, Wick P, Nowack B. Placing nanoplastics in the context of global plastic pollution. Nat Nanotechnol. 2021; 16(5):491–500. PMID: 33927363.6. Wright SL, Kelly FJ. Plastic and human health: a micro issue? Environ Sci Technol. 2017; 51(12):6634–6647. PMID: 28531345.7. Lu K, Zhan D, Fang Y, Li L, Chen G, Chen S, et al. Microplastics, potential threat to patients with lung diseases. Front Toxicol. 2022; 4:958414. PMID: 36245793.8. Okamura T, Hamaguchi M, Hasegawa Y, Hashimoto Y, Majima S, Senmaru T, et al. Oral exposure to polystyrene microplastics of mice on a normal or high-fat diet and intestinal and metabolic outcomes. Environ Health Perspect. 2023; 131(2):27006. PMID: 36821708.9. Park JH, Hong S, Kim OH, Kim CH, Kim J, Kim JW, et al. Polypropylene microplastics promote metastatic features in human breast cancer. Sci Rep. 2023; 13(1):6252. PMID: 37069244.10. Edaes FS, de Souza CB. BPS and BPF are as carcinogenic as BPA and are not viable alternatives for its replacement. Endocr Metab Immune Disord Drug Targets. 2022; 22(9):927–934. PMID: 35297356.11. Amran NH, Zaid SSM, Meng GY, Salleh A, Mokhtar MH. Protective role of kelulut honey against toxicity effects of polystyrene microplastics on morphology, hormones, and sex steroid receptor expression in the uterus of rats. Toxics. 2023; 11(4):324. PMID: 37112551.12. Wei Z, Wang Y, Wang S, Xie J, Han Q, Chen M. Comparing the effects of polystyrene microplastics exposure on reproduction and fertility in male and female mice. Toxicology. 2022; 465:153059. PMID: 34864092.13. Huang H, Wei F, Qiu S, Xing B, Hou J. Polystyrene microplastics trigger adiposity in mice by remodeling gut microbiota and boosting fatty acid synthesis. Sci Total Environ. 2023; 890:164297. PMID: 37211133.14. Zhao T, Shen L, Ye X, Bai G, Liao C, Chen Z, et al. Prenatal and postnatal exposure to polystyrene microplastics induces testis developmental disorder and affects male fertility in mice. J Hazard Mater. 2023; 445:130544. PMID: 36493639.15. Xu W, Yuan Y, Tian Y, Cheng C, Chen Y, Zeng L, et al. Oral exposure to polystyrene nanoplastics reduced male fertility and even caused male infertility by inducing testicular and sperm toxicities in mice. J Hazard Mater. 2023; 454:131470. PMID: 37116333.16. Hull MG, Glazener CM, Kelly NJ, Conway DI, Foster PA, Hinton RA, et al. Population study of causes, treatment, and outcome of infertility. Br Med J (Clin Res Ed). 1985; 291(6510):1693–1697.17. Gao HT, Xu R, Cao WX, Qian LL, Wang M, Lu L, et al. Effects of six priority controlled phthalate esters with long-term low-dose integrated exposure on male reproductive toxicity in rats. Food Chem Toxicol. 2017; 101:94–104. PMID: 28089693.18. Liu TM, Lee EH, Lim B, Shyh-Chang N. Concise review: balancing stem cell self-renewal and differentiation with PLZF. Stem Cells. 2016; 34(2):277–287. PMID: 26676652.19. Liu S, Tang Y, Chen B, Zhao Y, Aguilar ZP, Tao X, et al. Inhibition of testosterone synthesis induced by oral TiO2 NPs is associated with ROS-MAPK(ERK1/2)-StAR signaling pathway in SD rat. Toxicol Res (Camb). 2021; 10(4):937–946. PMID: 34484685.20. Mruk DD, Cheng CY. The mammalian blood-testis barrier: its biology and regulation. Endocr Rev. 2015; 36(5):564–591. PMID: 26357922.21. Lan Z, Yang WX. Nanoparticles and spermatogenesis: how do nanoparticles affect spermatogenesis and penetrate the blood-testis barrier. Nanomedicine (Lond). 2012; 7(4):579–596. PMID: 22471721.22. Li S, Wang Q, Yu H, Yang L, Sun Y, Xu N, et al. Polystyrene microplastics induce blood-testis barrier disruption regulated by the MAPK-Nrf2 signaling pathway in rats. Environ Sci Pollut Res Int. 2021; 28(35):47921–47931. PMID: 33895957.23. Dubey I, Khan S, Kushwaha S. Developmental and reproductive toxic effects of exposure to microplastics: a review of associated signaling pathways. Front Toxicol. 2022; 4:901798. PMID: 36119356.24. Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009; 1(6):a001651. PMID: 20457564.25. Arthur JS, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 2013; 13(9):679–692. PMID: 23954936.26. O’Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015; 66(1):311–328. PMID: 25587654.27. Cox KD, Covernton GA, Davies HL, Dower JF, Juanes F, Dudas SE. Human consumption of microplastics. Environ Sci Technol. 2019; 53(12):7068–7074. PMID: 31184127.28. Lenz R, Enders K, Nielsen TG. Microplastic exposure studies should be environmentally realistic. Proc Natl Acad Sci U S A. 2016; 113(29):E4121–E4122. PMID: 27407153.29. Prata JC, da Costa JP, Lopes I, Duarte AC, Rocha-Santos T. Environmental exposure to microplastics: an overview on possible human health effects. Sci Total Environ. 2020; 702:134455. PMID: 31733547.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Increase in Transepithelial Resistance Mouse Sertoli Cells by Leydig Cells Coculture
- Impact of polystyrene microplastic exposure on lipid profile and oxidative stress status of male and female Wistar rats
- Efficacy of In Vitro Germ Cell Culture in Nonobstructive Azoospermic Patients with Sertoli Cell Only Syndrome
- Chronic toxic effects of polystyrene microplastics on reproductive parameters of male rats
- Effect of Growth Hormone on Death of Sertoli Cell