J Korean Med Sci.
2024 Sep;39(36):e250. 10.3346/jkms.2024.39.e250.
Diagnostic Utility of Whole Genome Sequencing After Negative Karyotyping/Chromosomal Microarray in Infants Born With Multiple Congenital Anomalies
- Affiliations
-
- 1Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Cell and Gene Therapy Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 4Department of Laboratory Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 5Department of Pediatrics, Kangwon National University Hospital, Kangwon National University School of Medicine, Chuncheon, Korea
- 6Clinical Genomics Center, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 7Department of Pediatrics, CHA Gangnam Medical Center, CHA University, Seoul, Korea
- 8Division of Genome Science, Department of Precision Medicine, National Institute of Health, Cheongju, Korea
- 9National Institute of Health, Cheongju, Korea
- 10Department of Health Sciences and Technology, Samsung Advanced Institute for Health Sciences & Technology, Sungkyunkwan University, Seoul, Korea
- KMID: 2559773
- DOI: http://doi.org/10.3346/jkms.2024.39.e250
Abstract
- Background
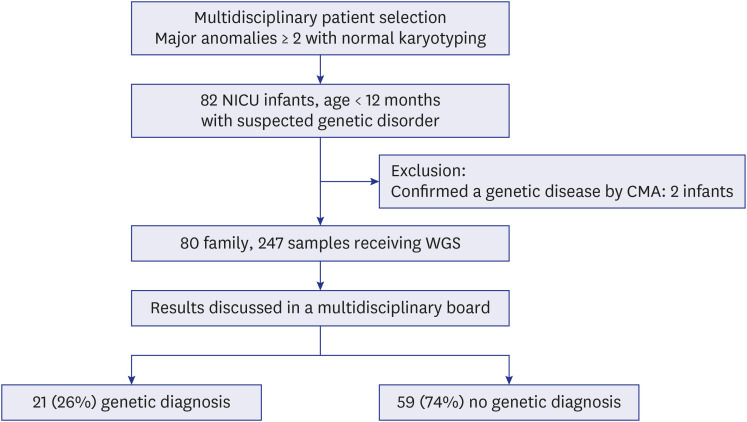
Achieving a definitive genetic diagnosis of unexplained multiple congenital anomalies (MCAs) in neonatal intensive care units (NICUs) infants is challenging because of the limited diagnostic capabilities of conventional genetic tests. Although the implementation of whole genome sequencing (WGS) has commenced for diagnosing MCAs, due to constraints in resources and faculty, many NICUs continue to utilize chromosomal microarray (CMA) and/or karyotyping as the initial diagnostic approach. We aimed to evaluate the diagnostic efficacy of WGS in infants with MCAs who have received negative results from karyotyping and/or CMA.
Methods
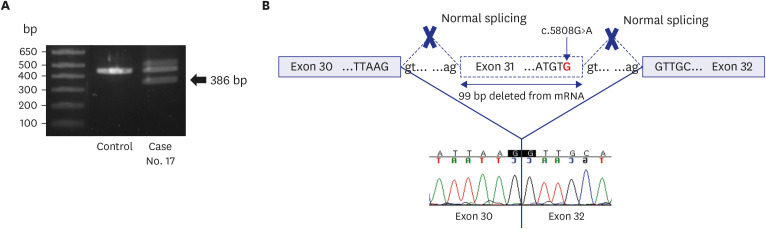
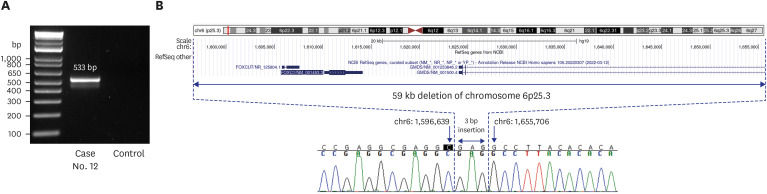
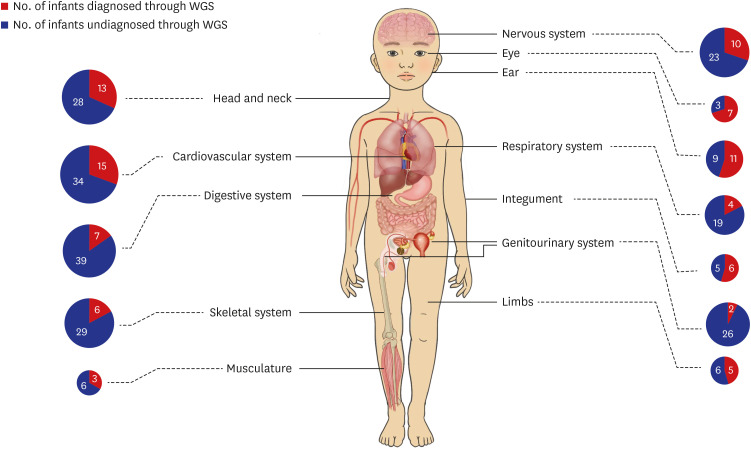
In this prospective study, we enrolled 80 infants with MCAs who were admitted to a NICU at a single center and had received negative results from CMA and/or karyotyping. The phenotypic characteristics were classified according to the International Classification of Diseases and the Human Phenotype Ontology. We assessed the diagnostic yield of trioWGS in infants with normal chromosomal result and explored the process of diagnosing by analyzing both phenotype and genotype. Also, we compared the phenotype and clinical outcomes between the groups diagnosed with WGS and the undiagnosed group. Results: The diagnostic yield of WGS was 26% (21/80), of which 76% were novel variants. There was a higher diagnostic yield in cases of craniofacial abnormalities, including those of the eye and ear, and a lower diagnostic yield in cases of gastrointestinal and genitourinary abnormalities. In addition, higher rates of rehabilitation therapy and gastrostomy were observed in WGS-diagnosed infants than in undiagnosed infants.
Conclusion
This prospective cohort study assessed the usefulness of trio-WGS following chromosomal analysis for diagnosing MCAs in the NICU and revealed improvements in the diagnostic yield and clinical utility of WGS.
Keyword
Figure
Reference
-
1. Marouane A, Olde Keizer RACM, Frederix GWJ, Vissers LELM, de Boode WP, van Zelst-Stams WAG. Congenital anomalies and genetic disorders in neonates and infants: a single-center observational cohort study. Eur J Pediatr. 2022; 181(1):359–367. PMID: 34347148.2. Agopian AJ, Evans JA, Lupo PJ. Analytic methods for evaluating patterns of multiple congenital anomalies in birth defect registries. Birth Defects Res. 2018; 110(1):5–11. PMID: 28925590.3. Jang W, Kim Y, Han E, Park J, Chae H, Kwon A, et al. Chromosomal microarray analysis as a first-tier clinical diagnostic test in patients with developmental delay/intellectual disability, autism spectrum disorders, and multiple congenital anomalies: a prospective multicenter study in Korea. Ann Lab Med. 2019; 39(3):299–310. PMID: 30623622.4. Miller DT, Adam MP, Aradhya S, Biesecker LG, Brothman AR, Carter NP, et al. Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am J Hum Genet. 2010; 86(5):749–764. PMID: 20466091.5. Kritioti E, Theodosiou A, Parpaite T, Alexandrou A, Nicolaou N, Papaevripidou I, et al. Unravelling the genetic causes of multiple malformation syndromes: a whole exome sequencing study of the Cypriot population. PLoS One. 2021; 16(7):e0253562. PMID: 34324503.6. NICUSeq Study Group. Krantz ID, Medne L, Weatherly JM, Wild KT, Biswas S, et al. Effect of whole-genome sequencing on the clinical management of acutely ill infants with suspected genetic disease: a randomized clinical trial. JAMA Pediatr. 2021; 175(12):1218–1226. PMID: 34570182.7. Elliott AM, du Souich C, Lehman A, Guella I, Evans DM, Candido T, et al. RAPIDOMICS: rapid genome-wide sequencing in a neonatal intensive care unit-successes and challenges. Eur J Pediatr. 2019; 178(8):1207–1218. PMID: 31172278.8. Olde Keizer RACM, Marouane A, Kerstjens-Frederikse WS, Deden AC, Lichtenbelt KD, Jonckers T, et al. Rapid exome sequencing as a first-tier test in neonates with suspected genetic disorder: results of a prospective multicenter clinical utility study in the Netherlands. Eur J Pediatr. 2023; 182(6):2683–2692. PMID: 36997769.9. Daoud H, Luco SM, Li R, Bareke E, Beaulieu C, Jarinova O, et al. Next-generation sequencing for diagnosis of rare diseases in the neonatal intensive care unit. CMAJ. 2016; 188(11):E254–E260. PMID: 27241786.10. Waggoner D, Wain KE, Dubuc AM, Conlin L, Hickey SE, Lamb AN, et al. Yield of additional genetic testing after chromosomal microarray for diagnosis of neurodevelopmental disability and congenital anomalies: a clinical practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2018; 20(10):1105–1113. PMID: 29915380.11. Jo HS, Yang M, Ahn SY, Sung SI, Park WS, Jang JH, et al. Optimal protocols and management of clinical and genomic data collection to assist in the early diagnosis and treatment of multiple congenital anomalies. Children (Basel). 2023; 10(10):1673. PMID: 37892336.12. Austin-Tse CA, Jobanputra V, Perry DL, Bick D, Taft RJ, Venner E, et al. Best practices for the interpretation and reporting of clinical whole genome sequencing. NPJ Genom Med. 2022; 7(1):27. PMID: 35395838.13. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; 17(5):405–424. PMID: 25741868.14. ClinGen Working Group. ClinGen sequence variant interpretation recommendation - version 1.0. Updated 2017. Accessed March 1, 2024. https://clinicalgenome.org/working-groups/sequence-variant-interpretation/ .15. Riggs ER, Andersen EF, Cherry AM, Kantarci S, Kearney H, Patel A, et al. Technical standards for the interpretation and reporting of constitutional copy-number variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics (ACMG) and the Clinical Genome Resource (ClinGen). Genet Med. 2020; 22(2):245–257. PMID: 31690835.16. Xiang Q, Chen J, Xiao X, Xu B, Xie H, Wang H, et al. Case report: The compound heterozygotes variants in FLT4 causes autosomal recessive hereditary lymphedema in a Chinese family. Front Genet. 2023; 14:1140406. PMID: 37035731.17. Kline AD, Moss JF, Selicorni A, Bisgaard AM, Deardorff MA, Gillett PM, et al. Diagnosis and management of Cornelia de Lange syndrome: first international consensus statement. Nat Rev Genet. 2018; 19(10):649–666. PMID: 29995837.18. Storm R, Cholewa-Waclaw J, Reuter K, Bröhl D, Sieber M, Treier M, et al. The bHLH transcription factor Olig3 marks the dorsal neuroepithelium of the hindbrain and is essential for the development of brainstem nuclei. Development. 2009; 136(2):295–305. PMID: 19088088.19. Müller T, Anlag K, Wildner H, Britsch S, Treier M, Birchmeier C. The bHLH factor Olig3 coordinates the specification of dorsal neurons in the spinal cord. Genes Dev. 2005; 19(6):733–743. PMID: 15769945.20. Lowenstein ED, Rusanova A, Stelzer J, Hernaiz-Llorens M, Schroer AE, Epifanova E, et al. Olig3 regulates early cerebellar development. eLife. 2021; 10:e64684. PMID: 33591268.21. Liu Z, Li H, Hu X, Yu L, Liu H, Han R, et al. Control of precerebellar neuron development by Olig3 bHLH transcription factor. J Neurosci. 2008; 28(40):10124–10133. PMID: 18829970.22. Wang H, Xiao F, Dong X, Lu Y, Cheng G, Wang L, et al. Diagnostic and clinical utility of next-generation sequencing in children born with multiple congenital anomalies in the China neonatal genomes project. Hum Mutat. 2021; 42(4):434–444. PMID: 33502061.23. Malinowski J, Miller DT, Demmer L, Gannon J, Pereira EM, Schroeder MC, et al. Systematic evidence-based review: outcomes from exome and genome sequencing for pediatric patients with congenital anomalies or intellectual disability. Genet Med. 2020; 22(6):986–1004. PMID: 32203227.24. Kingsmore SF, Cole FS. The role of genome sequencing in neonatal intensive care units. Annu Rev Genomics Hum Genet. 2022; 23(1):427–448. PMID: 35676073.25. Lionel AC, Costain G, Monfared N, Walker S, Reuter MS, Hosseini SM, et al. Improved diagnostic yield compared with targeted gene sequencing panels suggests a role for whole-genome sequencing as a first-tier genetic test. Genet Med. 2018; 20(4):435–443. PMID: 28771251.26. Halldorsson BV, Eggertsson HP, Moore KHS, Hauswedell H, Eiriksson O, Ulfarsson MO, et al. The sequences of 150,119 genomes in the UK Biobank. Nature. 2022; 607(7920):732–740. PMID: 35859178.27. Manning M, Hudgins L. Professional Practice and Guidelines Committee. Array-based technology and recommendations for utilization in medical genetics practice for detection of chromosomal abnormalities. Genet Med. 2010; 12(11):742–745. PMID: 20962661.28. Southard AE, Edelmann LJ, Gelb BD. Role of copy number variants in structural birth defects. Pediatrics. 2012; 129(4):755–763. PMID: 22430448.29. Clark MM, Stark Z, Farnaes L, Tan TY, White SM, Dimmock D, et al. Meta-analysis of the diagnostic and clinical utility of genome and exome sequencing and chromosomal microarray in children with suspected genetic diseases. NPJ Genom Med. 2018; 3(1):16. PMID: 30002876.30. Perovic D, Damnjanovic T, Jekic B, Dusanovic-Pjevic M, Grk M, Djuranovic A, et al. Chromosomal microarray in postnatal diagnosis of congenital anomalies and neurodevelopmental disorders in Serbian patients. J Clin Lab Anal. 2022; 36(6):e24441. PMID: 35441737.31. Kim YG, Kwon H, Park JH, Nam SH, Ha C, Shin S, et al. Whole-genome sequencing in clinically diagnosed Charcot-Marie-Tooth disease undiagnosed by whole-exome sequencing. Brain Commun. 2023; 5(3):fcad139. PMID: 37180992.32. Edgar AZ, Baby S. Axenfeld-Rieger syndrome. Updated October 24, 2022. Accessed March 1, 2024. https://www.ncbi.nlm.nih.gov/books/NBK538504/ .33. Haliburton GD, McKinsey GL, Pollard KS. Disruptions in a cluster of computationally identified enhancers near FOXC1 and GMDS may influence brain development. Neurogenetics. 2016; 17(1):1–9. PMID: 26382291.34. Yang L, Wei Z, Chen X, Hu L, Peng X, Wang J, et al. Use of medical exome sequencing for identification of underlying genetic defects in NICU: experience in a cohort of 2303 neonates in China. Clin Genet. 2022; 101(1):101–109. PMID: 34671977.35. Normand EA, Braxton A, Nassef S, Ward PA, Vetrini F, He W, et al. Clinical exome sequencing for fetuses with ultrasound abnormalities and a suspected Mendelian disorder. Genome Med. 2018; 10(1):74. PMID: 30266093.36. Dong X, Xiao T, Chen B, Lu Y, Zhou W. Precision medicine via the integration of phenotype-genotype information in neonatal genome project. Fundam Res. 2022; 2(6):873–884. PMID: 38933389.37. Harrison JE, Weber S, Jakob R, Chute CG. ICD-11: an international classification of diseases for the twenty-first century. BMC Med Inform Decis Mak. 2021; 21(Suppl 6):206. PMID: 34753471.38. Smith LD, Willig LK, Kingsmore SF. Whole-exome sequencing and whole-genome sequencing in critically ill neonates suspected to have single-gene disorders. Cold Spring Harb Perspect Med. 2015; 6(2):a023168. PMID: 26684335.39. Peterson B, Hernandez EJ, Hobbs C, Malone Jenkins S, Moore B, Rosales E, et al. Automated prioritization of sick newborns for whole genome sequencing using clinical natural language processing and machine learning. Genome Med. 2023; 15(1):18. PMID: 36927505.40. Mao D, Liu C, Wang L, Ai-Ouran R, Deisseroth C, Pasupuleti S, et al. AI-MARRVEL - a knowledge-driven AI system for diagnosing Mendelian disorders. NEJM AI. 2024; 1(5):10.1056/aioa2300009.41. Teo SM, Pawitan Y, Ku CS, Chia KS, Salim A. Statistical challenges associated with detecting copy number variations with next-generation sequencing. Bioinformatics. 2012; 28(21):2711–2718. PMID: 22942022.42. Kim DH, Yang M, Jo HS, Park J, Jang J, Shin S, et al. A preterm infant with feeding aspiration diagnosed with BOR syndrome, confirmed case by whole-genome sequencing and structural variant calling. Children (Basel). 2022; 10(1):76. PMID: 36670626.43. Hou L, Du Y, Zhang M, Su P, Zhao C, Wu Y. Novel mutations of PKHD1 and AHI1 identified in two families with cystic renal disease. Int J Clin Exp Pathol. 2018; 11(5):2869–2874. PMID: 31938409.44. Tartaglia M, Martinelli S, Stella L, Bocchinfuso G, Flex E, Cordeddu V, et al. Diversity and functional consequences of germline and somatic PTPN11 mutations in human disease. Am J Hum Genet. 2006; 78(2):279–290. PMID: 16358218.45. Duijkers FA, McDonald A, Janssens GE, Lezzerini M, Jongejan A, van Koningsbruggen S, et al. HNRNPR variants that impair homeobox gene expression drive developmental disorders in humans. Am J Hum Genet. 2019; 104(6):1040–1059. PMID: 31079900.46. Koide T, Odani S, Takahashi K, Ono T, Sakuragawa N. Antithrombin III Toyama: replacement of arginine-47 by cysteine in hereditary abnormal antithrombin III that lacks heparin-binding ability. Proc Natl Acad Sci U S A. 1984; 81(2):289–293. PMID: 6582486.47. Chen W, Zhang Y, Shen L, Zhu J, Cai K, Lu Z, et al. Biallelic DNAH9 mutations are identified in Chinese patients with defective left-right patterning and cilia-related complex congenital heart disease. Hum Genet. 2022; 141(8):1339–1353. PMID: 35050399.48. Janssen N, Bergman JE, Swertz MA, Tranebjaerg L, Lodahl M, Schoots J, et al. Mutation update on the CHD7 gene involved in CHARGE syndrome. Hum Mutat. 2012; 33(8):1149–1160. PMID: 22461308.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chromosomal Microarray Testing in 42 Korean Patients with Unexplained Developmental Delay, Intellectual Disability, Autism Spectrum Disorders, and Multiple Congenital Anomalies
- Prenatal chromosomal microarray analysis of fetus with increased nuchal translucency
- Clinical application of prenatal chromosomal microarray
- Clinical Applications of Chromosomal Microarray Analysis
- A clinical study of congenital anomalies in births associated with hydramnios