J Stroke.
2024 Sep;26(3):458-462. 10.5853/jos.2024.01529.
Neuroprotective Effects of Pulsed Electromagnetic Fields in Acute Stroke
- Affiliations
-
- 1Department of Medicine and Surgery, Unit of Neurology, Neurophysiology, Neurobiology and Psychiatry, Università Campus Bio-Medico di Roma, Roma, Italy
- 2Fondazione Policlinico Universitario Campus Bio-Medico, Roma, Italy
- 3IRCCS Istituto delle Scienze Neurologiche di Bologna, Department of Neurology and Stroke Center, Maggiore Hospital, Bologna, Italy
- 4Neurology Unit, Neuromotor & Rehabilitation Department, Azienda USL-IRCCS di Reggio Emilia, Reggio Emilia, Italy
- 5Stroke Unit, Tor Vergata Polyclinic Hospital, Tor Vergata University, Roma, Italy
- 6Department of Neuroscience and Rehabilitation, Division of Neurology, University Hospital of Ferrara, Ferrara, Italy
- 7University Vita-Salute San Raffaele, Milan, Italy
- 8Experimental Neurophysiology Unit, Institute of Experimental Neurology, Scientific Institute San Raffaele, Milan, Italy
- 9Department of Neurorehabilitation Sciences, Casa di Cura Igea, Milan, Italy
- 10Department of Information Engineering, Electronics and Telecommunications (DIET), Sapienza University of Rome, Roma, Italy
- 11Medical Division, IGEA, Carpi, Italy
- KMID: 2559565
- DOI: http://doi.org/10.5853/jos.2024.01529
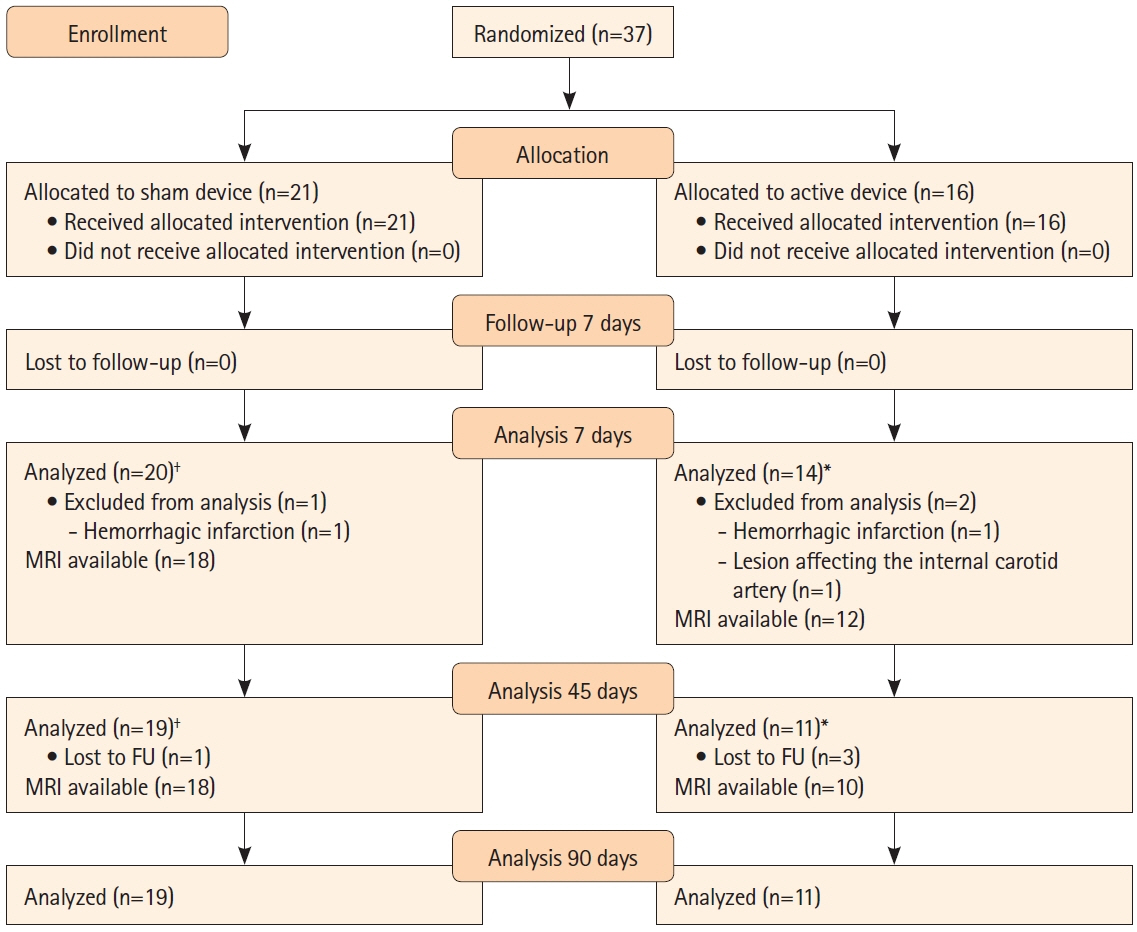
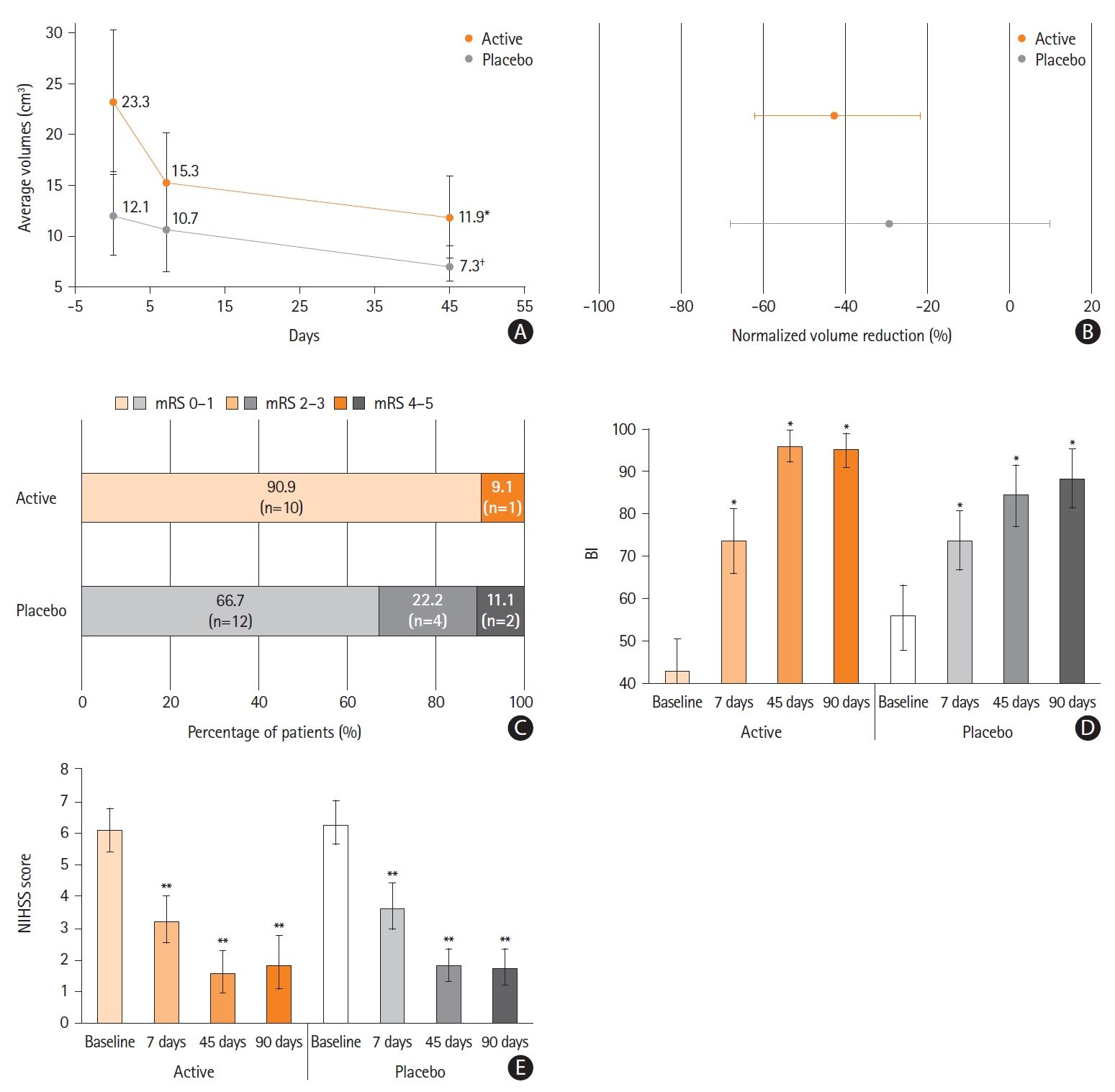
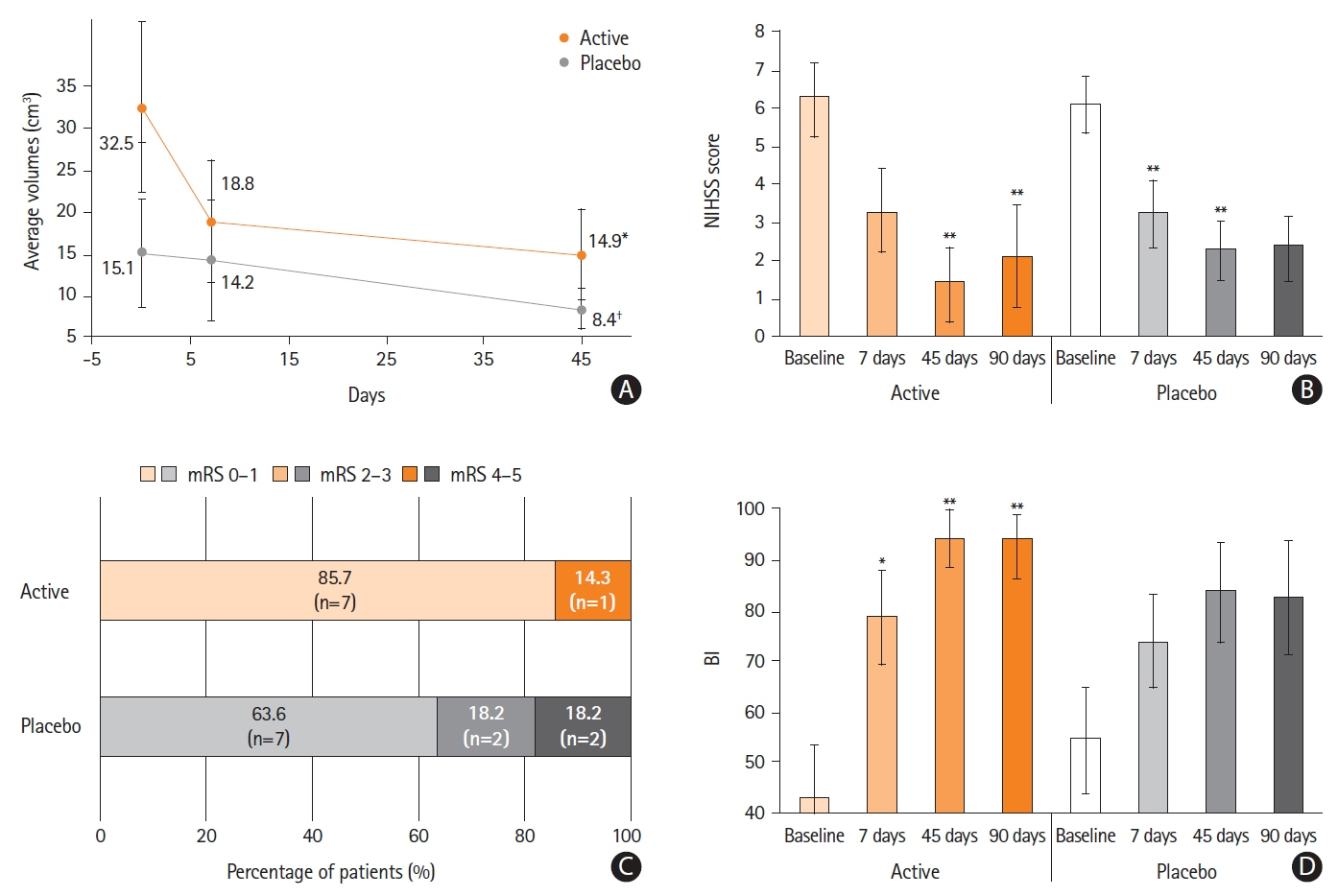
Figure
Reference
-
References
1. Chamorro Á, Dirnagl U, Urra X, Planas AM. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016; 15:869–881.2. Borea PA, Gessi S, Merighi S, Varani K. Adenosine as a multisignalling guardian angel in human diseases: when, where and how does it exert its protective effects? Trends Pharmacol Sci. 2016; 37:419–434.3. Capone F, Salati S, Vincenzi F, Liberti M, Aicardi G, Apollonio F, et al. Pulsed electromagnetic fields: a novel attractive therapeutic opportunity for neuroprotection after acute cerebral ischemia. Neuromodulation. 2022; 25:1240–1247.4. Melani A, Corti F, Cellai L, Vannucchi MG, Pedata F. Low doses of the selective adenosine A2A receptor agonist CGS21680 are protective in a rat model of transient cerebral ischemia. Brain Res. 2014; 1551:59–72.
Article5. Choi IY, Lee JC, Ju C, Hwang S, Cho GS, Lee HW, et al. A3 adenosine receptor agonist reduces brain ischemic injury and inhibits inflammatory cell migration in rats. Am J Pathol. 2011; 179:2042–2052.6. Grant G, Cadossi R, Steinberg G. Protection against focal cerebral ischemia following exposure to a pulsed electromagnetic field. Bioelectromagnetics. 1994; 15:205–216.7. Varani K, Gessi S, Merighi S, Iannotta V, Cattabriga E, Spisani S, et al. Effect of low frequency electromagnetic fields on A2A adenosine receptors in human neutrophils. Br J Pharmacol. 2002; 136:57–66.8. Capone F, Liberti M, Apollonio F, Camera F, Setti S, Cadossi R, et al. An open-label, one-arm, dose-escalation study to evaluate safety and tolerability of extremely low frequency magnetic fields in acute ischemic stroke. Sci Rep. 2017; 7:12145.
Article9. Colella M, Camera F, Capone F, Setti S, Cadossi R, Di Lazzaro V, et al. Patient semi-specific computational modeling of electromagnetic stimulation applied to neuroprotective treatments in acute ischemic stroke. Sci Rep. 2020; 10:2945.10. Capone F, Dileone M, Profice P, Pilato F, Musumeci G, Minicuci G, et al. Does exposure to extremely low frequency magnetic fields produce functional changes in human brain? J Neural Transm (Vienna). 2009; 116:257–265.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effects of static magnetic field and pulsed electromagnetic field on alkaline phosphatase and dna synthetic activity of ME3t3-E1 cells
- An experimental study on the effect of half sine-wave pulsed electromagnetic field in orthodontic tooth movement
- A Study Of Effect Of Pulsed Electromagnetic Fields On Osteogenesis In Rabbit Cranial Bone Defect
- The Effectiveness of Pulsed Electromagnetic Fields Therapy for Treatment of Chronic Pain
- Effects of electromagnetic stimulation on neurogenesis and neuronal proliferation in rat hippocampal slice culture