J Stroke.
2024 Sep;26(3):403-414. 10.5853/jos.2024.02250.
Early Neurological Deterioration and Time to Start Dual Antiplatelet Therapy in Patients With Acute Mild-to-Moderate Ischemic Stroke: A Pre-Specified Post Hoc Analysis of the ATAMIS Trial
- Affiliations
-
- 1Department of Neurology, General Hospital of Northern Theater Command, Shenyang, China
- KMID: 2559558
- DOI: http://doi.org/10.5853/jos.2024.02250
Abstract
- Background and Purpose
This study comprised a post hoc analysis of the Antiplatelet Therapy in Acute Mild to Moderate Ischemic Stroke (ATAMIS) trial aiming to determine whether the effect of dual antiplatelet therapy compared with that of monotherapy on preventing early neurological deterioration (END) differed according to the time from stroke onset to antiplatelet therapy (OTT).
Methods
In the ATAMIS trial, patients were divided into two subgroups: OTT from 0 to 24 hours (0–24 h group) and OTT from 24 to 48 hours (24–48 h group). We conducted multivariate regression analysis with continuous and categorical OTT to detect the effect of antiplatelet therapy. The primary outcome was END at 7 days, defined as an increase in the National Institutes of Health Stroke Scale (NIHSS) score of more than two points compared with the baseline. The safety outcomes were bleeding events and intracranial hemorrhage within 90 days.
Results
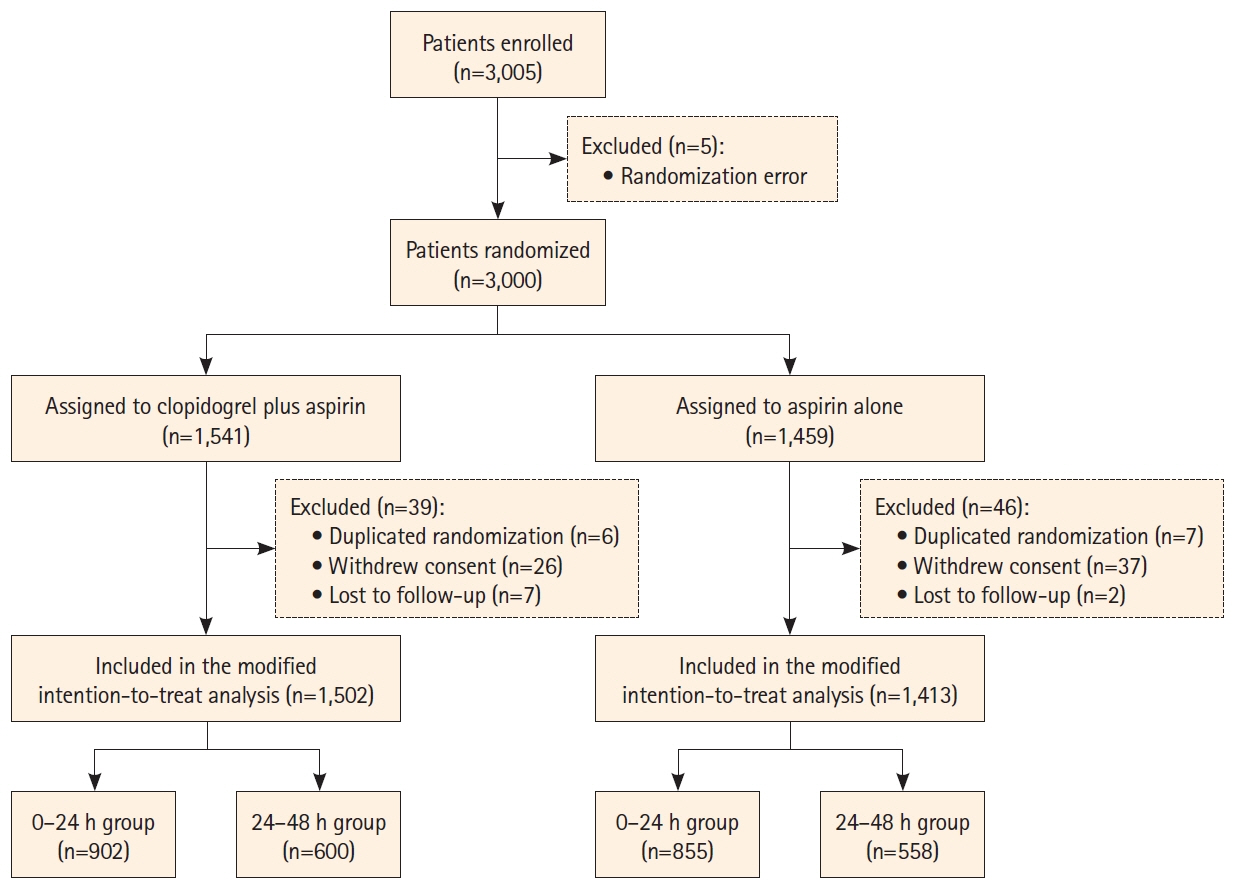
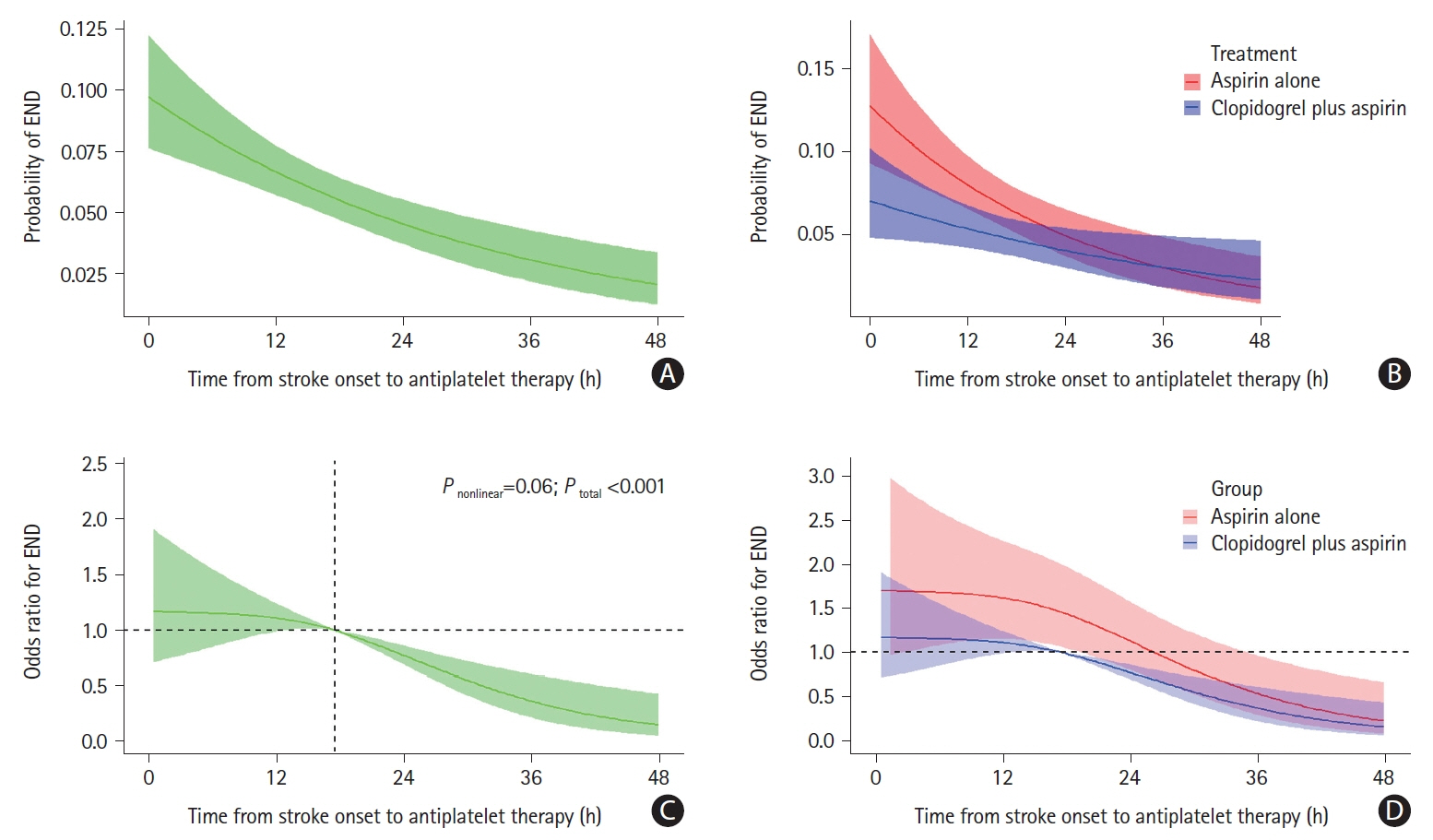
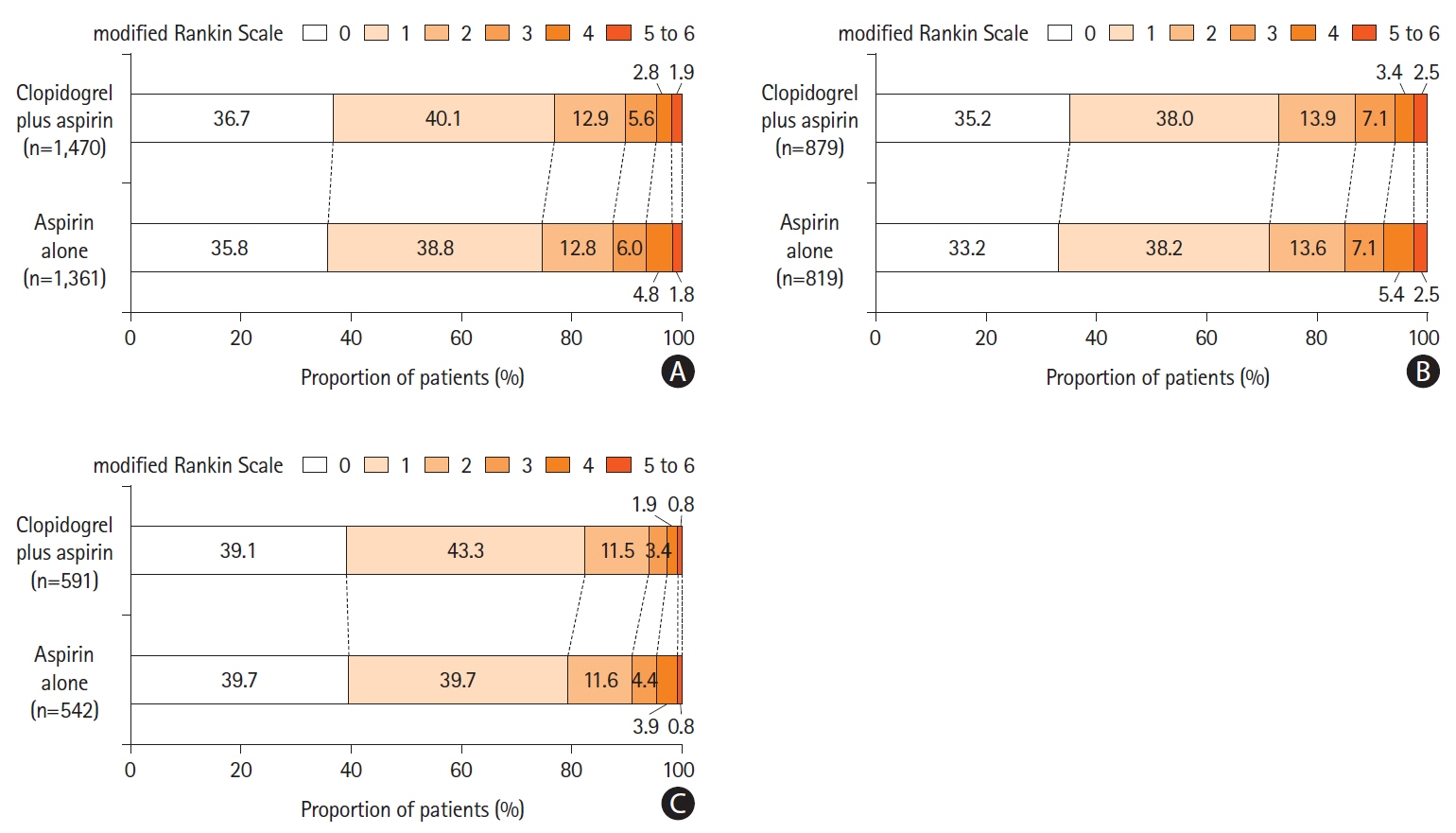
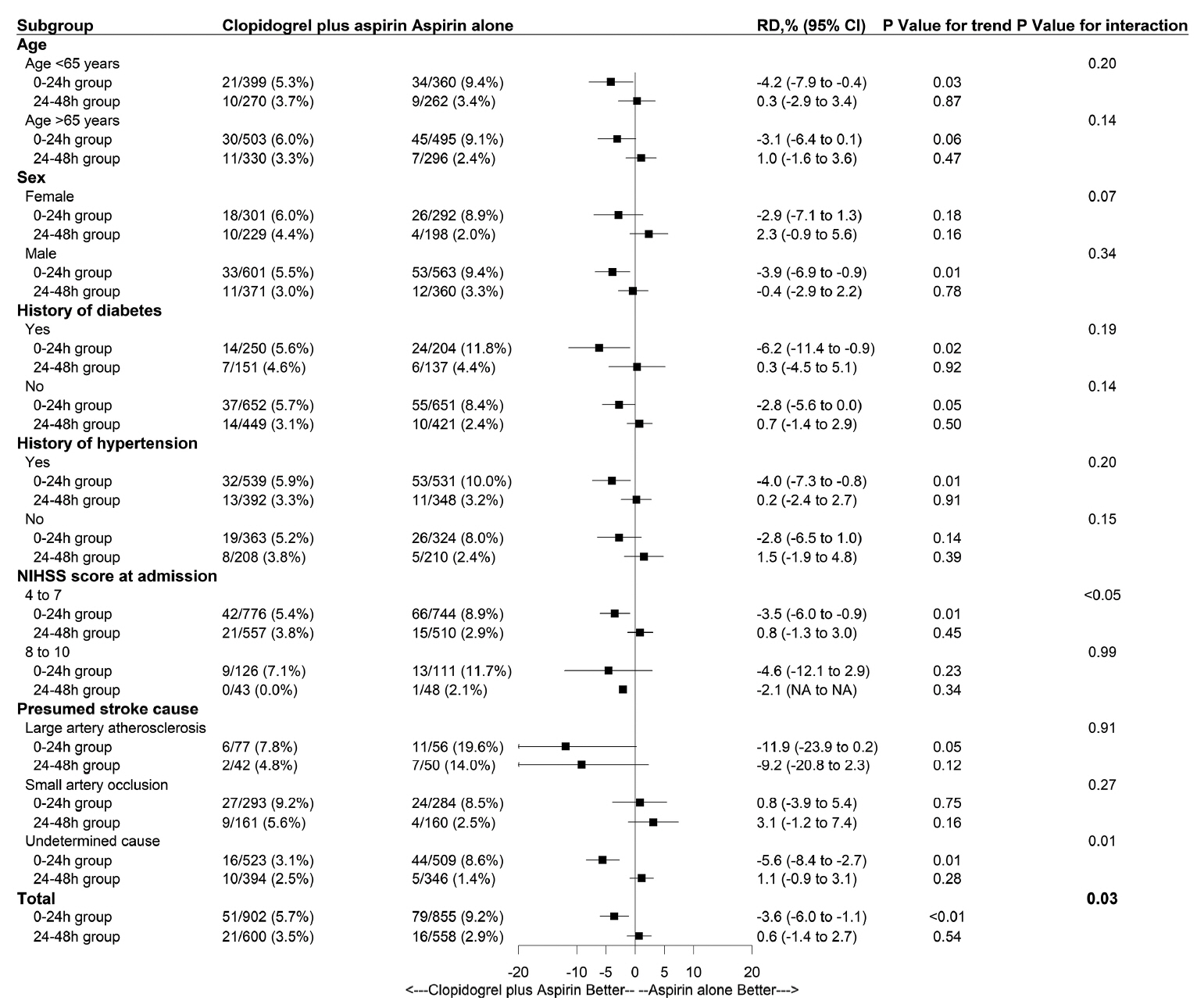
A total of 2,915 patients were included. With respect to END at 7 days, clopidogrel plus aspirin showed a lower proportion than aspirin alone across continuous OTT (4.8% vs. 6.7%; adjusted risk difference, -1.9%; 95% confidence interval [CI], -3.6% to -0.2%; P=0.03), and was lower in the 0–24 hours group (5.7% vs. 9.2%; adjusted risk difference, -3.7%; 95% CI, -5.5% to -2.0%; P<0.01), but similar in the 24–48 hours group (3.5% vs. 2.9%; adjusted risk difference, 0.6%; 95% CI, -0.8% to 2.0%; P=0.40). We identified a significant interaction between the treatment effect and time subgroup with respect to the primary outcome (P=0.03). The occurrence of bleeding events and intracranial hemorrhage was similar in the time subgroup.
Conclusion
For patients with acute mild-to-moderate ischemic stroke, clopidogrel plus aspirin was associated with a lower risk of END at 7 days than aspirin alone when it was started within 24 hours of symptom onset.
Keyword
Figure
Reference
-
References
1. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019; 50:e344–e418.
Article2. Seker F, Qureshi MM, Möhlenbruch MA, Nogueira RG, Abdalkader M, Ribo M, et al. Reperfusion without functional independence in late presentation of stroke with large vessel occlusion. Stroke. 2022; 53:3594–3604.
Article3. Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013; 369:11–19.
Article4. Johnston SC, Easton JD, Farrant M, Barsan W, Conwit RA, Elm JJ, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018; 379:215–225.
Article5. Gao Y, Chen W, Pan Y, Jing J, Wang C, Johnston SC, et al. Dual antiplatelet treatment up to 72 hours after ischemic stroke. N Engl J Med. 2023; 389:2413–2424.6. Wasserman JK, Perry JJ, Sivilotti ML, Sutherland J, Worster A, Émond M, et al. Computed tomography identifies patients at high risk for stroke after transient ischemic attack/nondisabling stroke: prospective, multicenter cohort study. Stroke. 2015; 46:114–119.
Article7. Chen HS, Cui Y, Wang XH, Ma YT, Han J, Duan YJ, et al. Clopidogrel plus aspirin vs aspirin alone in patients with acute mild to moderate stroke: the ATAMIS randomized clinical trial. JAMA Neurol. 2024; 81:450–460.
Article8. Kheiri B, Osman M, Abdalla A, Haykal T, Swaid B, Ahmed S, et al. Clopidogrel and aspirin after ischemic stroke or transient ischemic attack: an updated systematic review and metaanalysis of randomized clinical trials. J Thromb Thrombolysis. 2019; 47:233–247.
Article9. Hou X, Li X, Wang X, Chen H. Antiplatelet therapy in acute mild-moderate ischemic stroke (ATAMIS): a parallel, randomised, open-label, multicentre, prospective study. Stroke Vasc Neurol. 2018; 3:263–267.
Article10. Yi X, Zhou Q, Wang C, Lin J, Chai Z. Aspirin plus clopidogrel may reduce the risk of early neurologic deterioration in ischemic stroke patients carrying CYP2C19*2 reduced-function alleles. J Neurol. 2018; 265:2396–2403.11. Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013; 44:2064–2089.
Article12. Guo C, Song J, Li L, Yang J, Huang J, Xie D, et al. Association of procedure time with clinical and procedural outcome in patients with basilar occlusion undergoing embolectomy. Neurology. 2023; 101:e253–e266.
Article13. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993; 24:35–41.
Article14. Liu H, Liu K, Zhang K, Zong C, Yang H, Li Y, et al. Early neurological deterioration in patients with acute ischemic stroke: a prospective multicenter cohort study. Ther Adv Neurol Disord. 2023; 16:17562864221147743.
Article15. Seners P, Turc G, Oppenheim C, Baron JC. Incidence, causes and predictors of neurological deterioration occurring within 24 h following acute ischaemic stroke: a systematic review with pathophysiological implications. J Neurol Neurosurg Psychiatry. 2015; 86:87–94.16. Saver JL, Fonarow GC, Smith EE, Reeves MJ, Grau-Sepulveda MV, Pan W, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013; 309:2480–2488.
Article17. Matsuo R, Yamaguchi Y, Matsushita T, Hata J, Kiyuna F, Fukuda K, et al. Association between onset-to-door time and clinical outcomes after ischemic stroke. Stroke. 2017; 48:3049–3056.
Article18. Seners P, Yuen N, Mlynash M, Snyder SJ, Heit JJ, Lansberg MG, et al. Quantification of penumbral volume in association with time from stroke onset in acute ischemic stroke with large vessel occlusion. JAMA Neurol. 2023; 80:523–528.
Article19. Simonsen CZ, Schmitz ML, Madsen MH, Mikkelsen IK, Chandra RV, Leslie-Mazwi T, et al. Early neurological deterioration after thrombolysis: clinical and imaging predictors. Int J Stroke. 2016; 11:776–782.
Article20. Johnston SC, Elm JJ, Easton JD, Farrant M, Barsan WG, Kim AS, et al. Time course for benefit and risk of clopidogrel and aspirin after acute transient ischemic attack and minor ischemic stroke. Circulation. 2019; 140:658–664.21. Kim JT, Lee JS, Kim BJ, Park JM, Kang K, Lee SJ, et al. Frequency, management, and outcomes of early neurologic deterioration due to stroke progression or recurrence. J Stroke Cerebrovasc Dis. 2023; 32:106940.
Article22. Thanvi B, Treadwell S, Robinson T. Early neurological deterioration in acute ischaemic stroke: predictors, mechanisms and management. Postgrad Med J. 2008; 84:412–417.
Article23. Li W, Lin L, Zhang M, Wu Y, Liu C, Li X, et al. Safety and preliminary efficacy of early tirofiban treatment after alteplase in acute ischemic stroke patients. Stroke. 2016; 47:2649–2651.
Article24. Liu J, Shi Q, Sun Y, He J, Yang B, Zhang C, et al. Efficacy of tirofiban administered at different time points after intravenous thrombolytic therapy with alteplase in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. 2019; 28:1126–1132.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antiplatelet Therapy for Secondary Stroke Prevention in Patients with Ischemic Stroke or Transient Ischemic Attack
- Dual Antiplatelet Therapy after Noncardioembolic Ischemic Stroke or Transient Ischemic Attack: Pros and Cons
- Induced Hypertensive Therapy in an Acute Ischemic Stroke Patient with Early Neurological Deterioration
- Low-Molecular-Weight Heparin or Dual Antiplatelet Therapy Is More Effective Than Aspirin Alone in Preventing Early Neurological Deterioration and Improving the 6-Month Outcome in Ischemic Stroke Patients
- Review of Updated Guidelines and Evidence for Antithrombotic Therapy in Acute Ischemic Stroke