J Liver Cancer.
2024 Sep;24(2):234-242. 10.17998/jlc.2024.05.23.
Cure can be achieved by conversion to microwave ablation following atezolizumab-bevacizumab therapy in unresectable hepatocellular carcinoma
- Affiliations
-
- 1Section of Gastroenterology, University of Santo Tomas Hospital, Manila, Philippines
- KMID: 2559470
- DOI: http://doi.org/10.17998/jlc.2024.05.23
Abstract
- Backgrounds/Aims
Atezolizumab/bevacizumab is the recommended first-line systemic therapy for unresectable hepatocellular carcinoma (uHCC) and may facilitate curative conversion through resection and locoregional therapies. However, there have been very few reports on curative conversion using microwave ablation (MWA). This study aimed to determine the curative conversion rate with MWA using atezolizumab-bevacizumab as the first-line treatment in patients with uHCC, and to compare the characteristics and survival of patients with and without curative conversion.
Methods
Consecutive patients with uHCC who were started on atezolizumab-bevacizumab from May 2021 to December 2023 in a single tertiary center were included. Objective response rate (ORR) and disease control rate (DCR) were based on the Response Evaluation Criteria In Solid Tumors (RECIST) 1.1 and modified RECIST (mRECIST) criteria.
Results
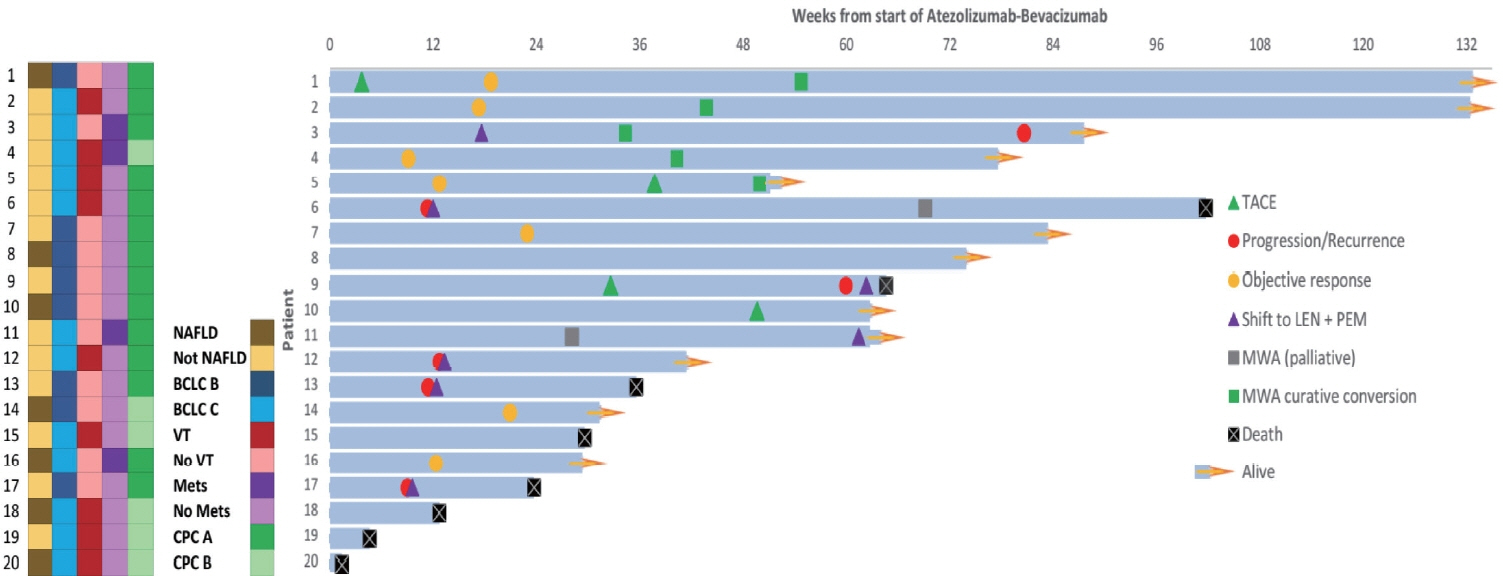
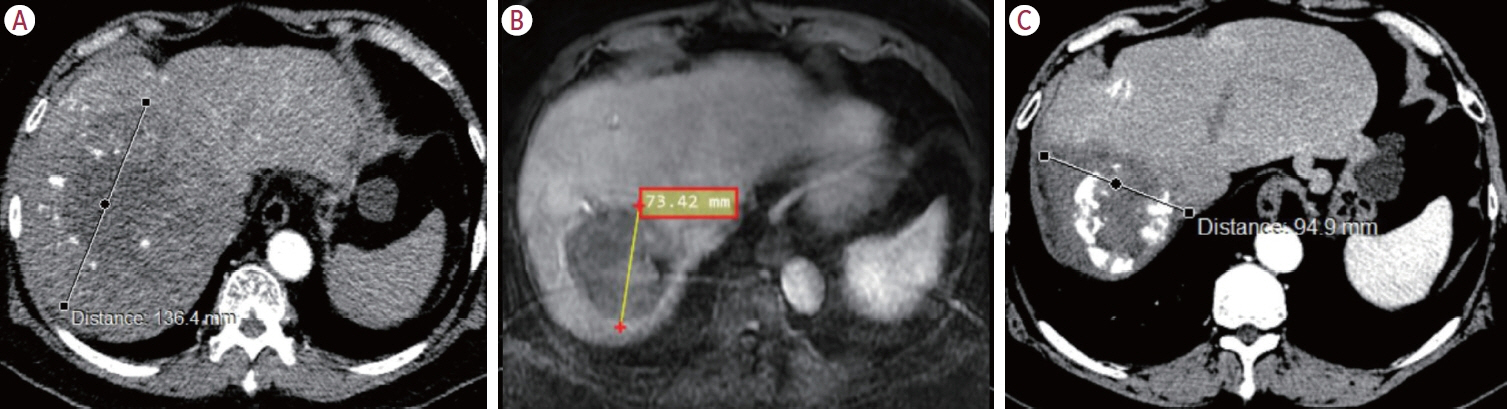
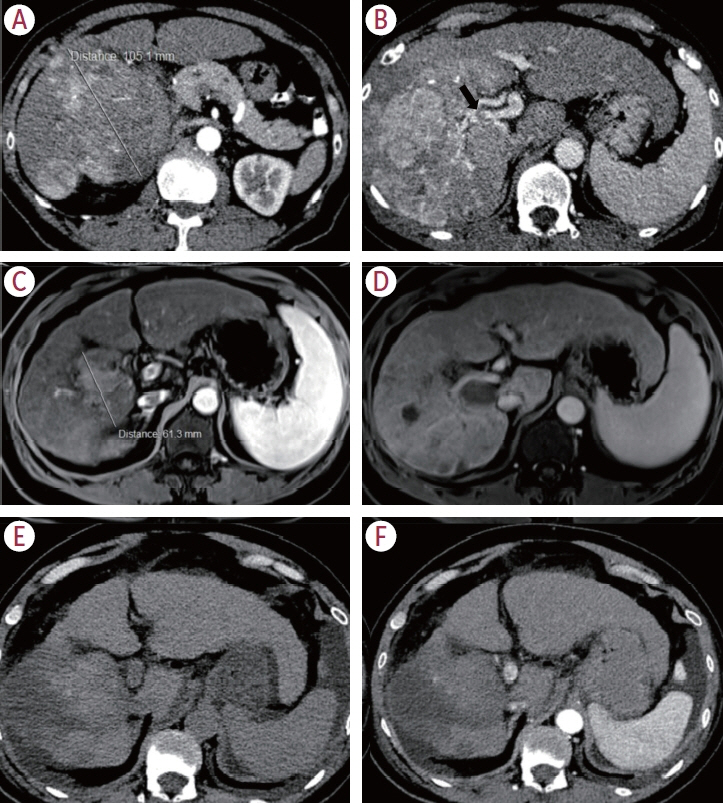
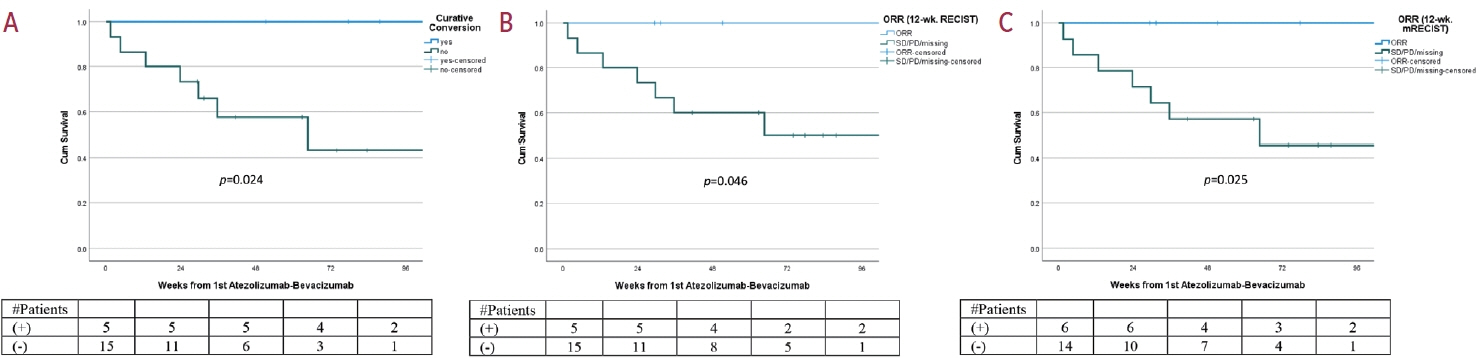
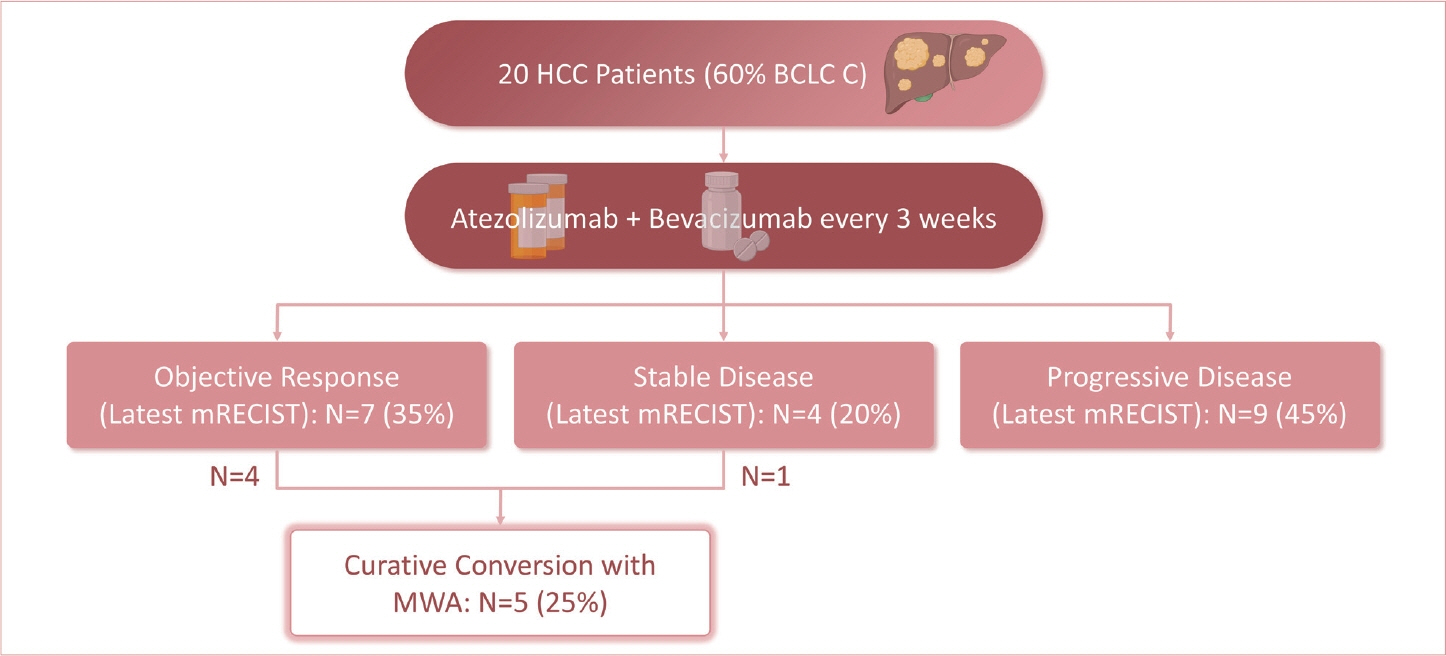
Twenty consecutive patients with uHCC (60% advanced-stage) were included, 90% exceeding the up-to-7 criteria. The ORR and DCR were 35% and 60%, 35% and 55% using RECIST and mRECIST, respectively. Five patients (25%) underwent successful curative conversion with MWA (four advanced and one intermediate stage) despite a median HCC size of 6.1 cm (range, 2.4-7.3). Two of these patients were tumor and drug-free 132-133 weeks from the 1st atezolizumab-bevacizumab dose. Patients who underwent curative conversion had significantly longer survival than those who did not (P=0.024). Other factors associated with survival were male sex, Child-Pugh class A, and an objective response.
Conclusions
Despite the relatively large tumor size, successful curative conversion with MWA was achieved with first-line atezolizumab-bevacizumab in uHCC. However, data from prospective multicenter trials are required to determine whether this strategy is universally applicable.
Keyword
Figure
Reference
-
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–249.2. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020; 38:2960–2970.3. Abou-Alfa GK, Chan SL, Kudo M, Lau G, Kelley RK, Furuse J, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022; 40 Suppl 4:379.4. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020; 382:1894–1905.
Article5. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–390.
Article6. Mazzaferro V, Citterio D, Bhoori S, Bongini M, Miceli R, De Carlis L, et al. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): a randomised, controlled, phase 2b/3 trial. Lancet Oncol. 2020; 21:947–956.
Article7. Li W, Pei Y, Wang Z, Liu J. Efficacy of transarterial chemoembolization monotherapy or combination conversion therapy in unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Front Oncol. 2022; 12:930868.
Article8. Kudo M. Atezolizumab plus bevacizumab followed by curative conversion (ABC conversion) in patients with unresectable, TACE-unsuitable intermediate-stage hepatocellular carcinoma. Liver Cancer. 2022; 11:399–406.9. Zhang W, Hu B, Han J, Wang Z, Ma G, Ye H, et al. Surgery after conversion therapy with PD-1 inhibitors plus tyrosine kinase inhibitors are effective and safe for advanced hepatocellular carcinoma: a pilot study of ten patients. Front Oncol. 2021; 11:747950.
Article10. Kudo M, Aoki T, Ueshima K, Tsuchiya K, Morita M, Chishina H, et al. Achievement of complete response and drug-free status by atezolizumab plus bevacizumab combined with or without curative conversion in patients with transarterial chemoembolization-unsuitable, intermediate-stage hepatocellular carcinoma: a multicenter proof-of-concept study. Liver Cancer. 2023; 12:321–338.
Article11. Kudo M. A novel treatment strategy for patients with intermediate-stage HCC who are not suitable for TACE: upfront systemic therapy followed by curative conversion. Liver Cancer. 2021; 10:539–544.
Article12. Zhang W, Tong S, Hu B, Wan T, Tang H, Zhao F, et al. Lenvatinib plus antiPD-1 antibodies as conversion therapy for patients with unresectable intermediate-advanced hepatocellular carcinoma: a single-arm, phase II trial. J Immunother Cancer. 2023; 11:e007366.
Article13. Huang C, Zhu XD, Shen YH, Xu B, Wu D, Ji Y, et al. Radiographic and α-fetoprotein response predict pathologic complete response to immunotherapy plus a TKI in hepatocellular carcinoma: a multicenter study. BMC Cancer. 2023; 23:416.14. Tomonari T, Tani J, Sato Y, Tanaka H, Tanaka T, Taniguchi T, et al. Clinical features and outcomes of conversion therapy in patients with unresectable hepatocellular carcinoma. Cancers (Basel). 2023; 15:5221.
Article15. Tak WY, Lin SM, Wang Y, Zheng J, Vecchione A, Park SY, et al. Phase III HEAT study adding lyso-thermosensitive liposomal doxorubicin to radiofrequency ablation in patients with unresectable hepatocellular carcinoma lesions. Clin Cancer Res. 2018; 24:73–83.
Article16. Zhang NN, Lu W, Cheng XJ, Liu JY, Zhou YH, Li F. High-powered microwave ablation of larger hepatocellular carcinoma: evaluation of recurrence rate and factors related to recurrence. Clin Radiol. 2015; 70:1237–1243.
Article17. Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009; 10:35–43.
Article18. Lee MS, Ryoo BY, Hsu CH, Numata K, Stein S, Verret W, et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 2020; 21:808–820.19. Salem R, Li D, Sommer N, Hernandez S, Verret W, Ding B, et al. Characterization of response to atezolizumab + bevacizumab versus sorafenib for hepatocellular carcinoma: results from the IMbrave150 trial. Cancer Med. 2021; 10:5437–5447.
Article20. Zerbini A, Pilli M, Penna A, Pelosi G, Schianchi C, Molinari A, et al. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res. 2006; 66:1139–1146.
Article21. Leuchte K, Staib E, Thelen M, Gödel P, Lechner A, Zentis P, et al. Microwave ablation enhances tumor-specific immune response in patients with hepatocellular carcinoma. Cancer Immunol Immunother. 2021; 70:893–907.
Article22. Qin S, Chen M, Cheng AL, Kaseb AO, Kudo M, Lee HC, et al. Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave050): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023; 402:1835–1847.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Atezolizumab and bevacizumab for hepatocellular carcinoma: How to approach salvage therapy for non-responders?: Editorial on “Sorafenib vs. Lenvatinib in advanced hepatocellular carcinoma after atezolizumab/bevacizumab failure: A real-world study”
- A Case of Transverse Myelitis Following Treatment with Atezolizumab for Advanced Hepatocellular Carcinoma
- Complete response in hepatocellular carcinoma with lymph node metastasis by combination therapy of atezolizumab and bevacizumab: a case report
- Favorable response of hepatocellular carcinoma with portal vein tumor thrombosis after radiotherapy combined with atezolizumab plus bevacizumab
- Clinical significance of the discrepancy between radiological findings and biochemical responses in atezolizumab plus bevacizumab for hepatocellular carcinoma