J Liver Cancer.
2024 Sep;24(2):131-144. 10.17998/jlc.2024.08.04.
Local ablation for hepatocellular carcinoma: 2024 expert consensus-based practical recommendation of the Korean Liver Cancer Association
- Affiliations
-
- 1Department of Radiology, Samsung Medical Center, Seoul, Korea
- 2Department of Internal Medicine, Seoul St. Mary’s Hospital, The Catholic University of Korea, Seoul, Korea
- 3Department of Internal Medicine, Kyungpook National University Hospital, School of Medicine, Kyungpook National University, Daegu, Korea
- 4Department of Radiology, Chosun University Hospital, Chosun University College of Medicine, Gwangju, Korea
- 5Department of Radiology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 6Department of Radiology, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
- 7Department of Radiology, Yeouido St. Mary’s Hospital, The Catholic University of Korea, Seoul, Korea
- 8Department of Radiology, Research Institute of Radiological Science, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 9Department of Radiology, Soonchunhyang University Hospital Bucheon, Soonchunhyang University College of Medicine, Bucheon, Korea
- 10Department of Radiology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 11Department of Internal Medicine, Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- 12Department of Internal Medicine, Hallym University Sacred Heart Hospital, Anyang, Korea
- KMID: 2559461
- DOI: http://doi.org/10.17998/jlc.2024.08.04
Abstract
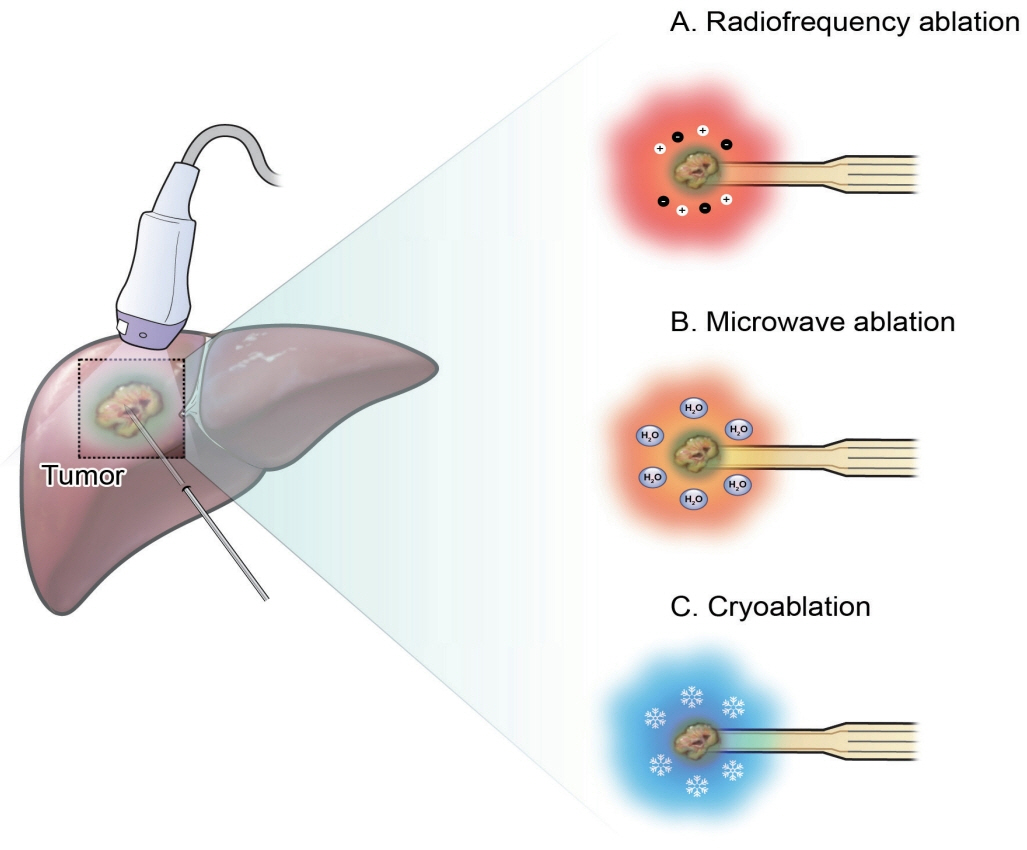
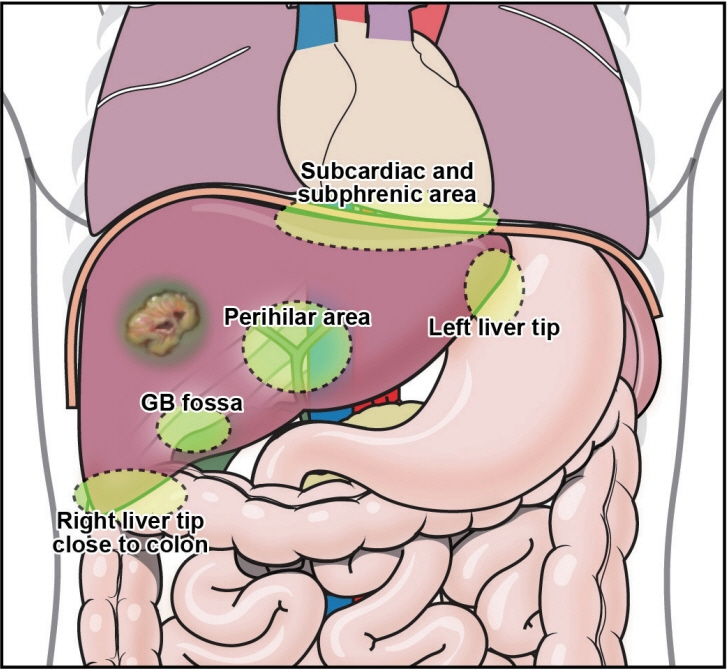
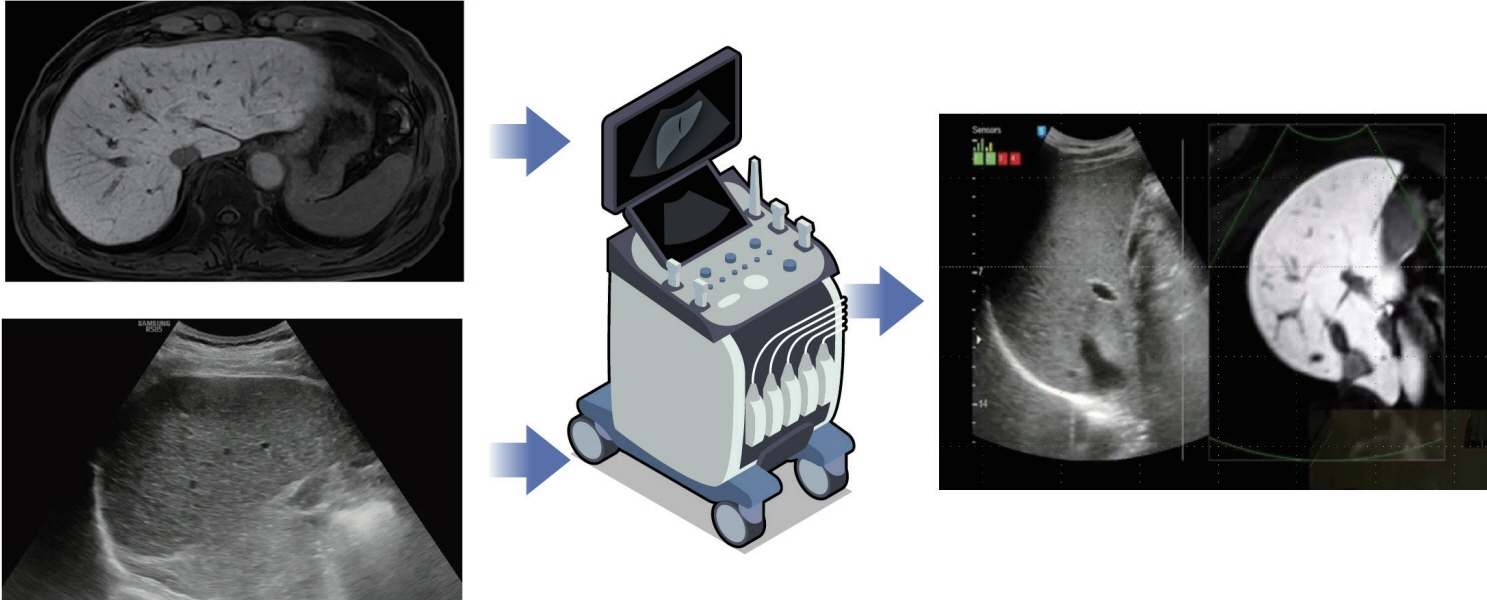
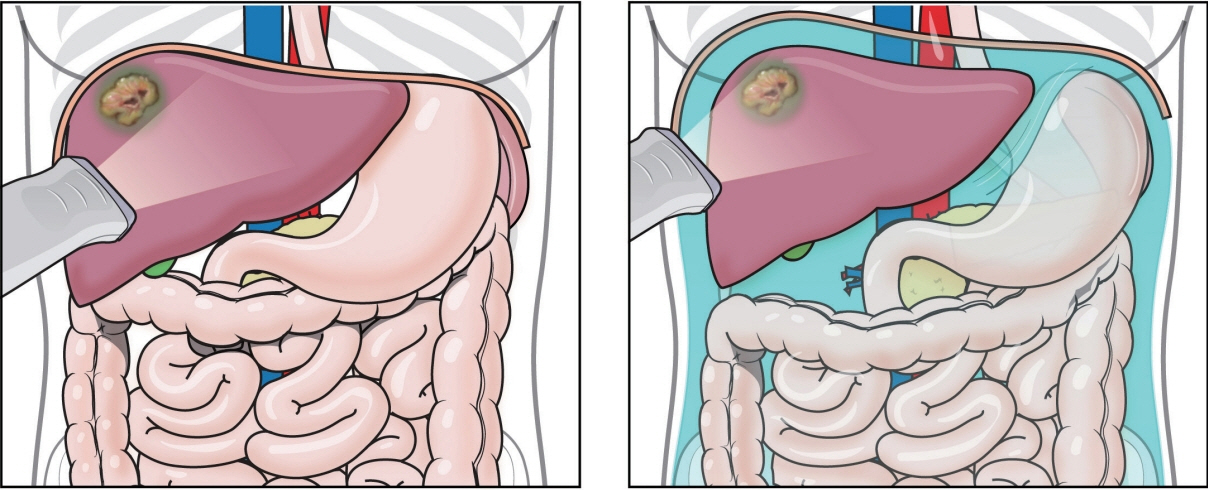
- Local ablation for hepatocellular carcinoma (HCC), a non-surgical option that directly targets and destroys tumor cells, has advanced significantly since the 1990s. Therapies with different energy sources, such as radiofrequency ablation, microwave ablation, and cryoablation, employ different mechanisms to induce tumor necrosis. The precision, safety, and effectiveness of these therapies have increased with advances in guiding technologies and device improvements. Consequently, local ablation has become the firstline treatment for early-stage HCC. The lack of organized evidence and expert opinions regarding patient selection, pre-procedure preparation, procedural methods, swift post-treatment evaluation, and follow-up has resulted in clinicians following varied practices. Therefore, an expert consensus-based practical recommendation for local ablation was developed by a group of experts in radiology and hepatology from the Research Committee of the Korean Liver Cancer Association in collaboration with the Korean Society of Image-guided Tumor Ablation to provide useful information and guidance for performing local ablation and for the pre- and posttreatment management of patients.
Keyword
Figure
Cited by 3 articles
-
Practical consensus multi-specialty guidelines on image-guided ablation for hepatocellular carcinoma
David S. Lu
J Liver Cancer. 2024;24(2):120-123. doi: 10.17998/jlc.2024.09.11.Evolving trends in epidemiology, etiology, and treatment patterns for hepatocellular carcinoma in South Korea
Soo Young Hwang, Ju Dong Yang
J Liver Cancer. 2025;25(1):4-6. doi: 10.17998/jlc.2024.12.04.Microwave ablation vs. liver resection for patients with hepatocellular carcinomas
Hyundam Gu, Yeonjoo Seo, Dong Jin Chung, Kwang Yeol Paik, Seung Kew Yoon, Jihye Lim
J Liver Cancer. 2025;25(1):99-108. doi: 10.17998/jlc.2025.02.02.
Reference
-
References
1. Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006; 243:321–328.
Article2. Feng K, Yan J, Li X, Xia F, Ma K, Wang S, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012; 57:794–802.
Article3. Wang Y, Luo Q, Li Y, Deng S, Wei S, Li X. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinomas: a metaanalysis of randomized and nonrandomized controlled trials. PLoS One. 2014; 9:e84484.
Article4. Korean Liver Cancer Association (KLCA); National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. J Liver Cancer. 2023; 23:1–120.5. Korean Liver Cancer Association (KLCA), National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Korean J Radiol. 2022; 23:1126–1240.6. Korean Liver Cancer Association (KLCA); National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol. 2022; 28:583–705.7. Lee DH, Lee JM, Lee JY, Kim SH, Han JK, Choi BI. Radiofrequency ablation for intrahepatic recurrent hepatocellular carcinoma: long-term results and prognostic factors in 168 patients with cirrhosis. Cardiovasc Intervent Radiol. 2014; 37:705–715.
Article8. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018; 69:182–236.9. European Association for the Study of the Liver. EASL clinical practice guidelines on prevention and management of bleeding and thrombosis in patients with cirrhosis. J Hepatol. 2022; 76:1151–1184.10. Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, et al. Earlystage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005; 234:961–967.
Article11. Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005; 129:122–130.
Article12. Luo W, Zhang Y, He G, Yu M, Zheng M, Liu L, et al. Effects of radiofrequency ablation versus other ablating techniques on hepatocellular carcinomas: a systematic review and meta-analysis. World J Surg Oncol. 2017; 15:126.
Article13. Violi NV, Duran R, Guiu B, Cercueil JP, Aubé C, Digklia A, et al. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018; 3:317–325.14. Gupta P, Maralakunte M, Kumar-M P, Chandel K, Chaluvashetty SB, Bhujade H, et al. Overall survival and local recurrence following RFA, MWA, and cryoablation of very early and early HCC: a systematic review and Bayesian network meta-analysis. Eur Radiol. 2021; 31:5400–5408.
Article15. Yu Q, Liu C, Navuluri R, Ahmed O. Percutaneous microwave ablation versus radiofrequency ablation of hepatocellular carcinoma: a meta-analysis of randomized controlled trials. Abdom Radiol (NY). 2021; 46:4467–4475.
Article16. Patel IJ, Rahim S, Davidson JC, Hanks SE, Tam AL, Walker TG, et al. Society of Interventional Radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions-part II: recommendations: endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol. 2019; 30:1168–1184. e1.17. Tripodi A, Primignani M, Mannucci PM, Caldwell SH. Changing concepts of cirrhotic coagulopathy. Am J Gastroenterol. 2017; 112:274–281.
Article18. Rhim H, Yoon KH, Lee JM, Cho Y, Cho JS, Kim SH, et al. Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiographics. 2003; 23:123–134. discussion 134-136.19. de Baère T, Risse O, Kuoch V, Dromain C, Sengel C, Smayra T, et al. Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR Am J Roentgenol. 2003; 181:695–700.
Article20. Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003; 228:235–240.
Article21. Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012; 107:569–577. quiz 578.
Article22. Kang TW, Lim HK, Lee MW, Kim YS, Rhim H, Lee WJ, et al. Aggressive intrasegmental recurrence of hepatocellular carcinoma after radiofrequency ablation: risk factors and clinical significance. Radiology. 2015; 276:274–285.
Article23. Lee S, Kang TW, Cha DI, Song KD, Lee MW, Rhim H, et al. Radiofrequency ablation vs. surgery for perivascular hepatocellular carcinoma: Propensity score analyses of long-term outcomes. J Hepatol. 2018; 69:70–78.24. Song KD, Lim HK, Rhim H, Lee MW, Kang TW, Paik YH, et al. Hepatic resection vs percutaneous radiofrequency ablation of hepatocellular carcinoma abutting right diaphragm. World J Gastrointest Oncol. 2019; 11:227–237.
Article25. Lee MW, Kang D, Lim HK, Cho J, Sinn DH, Kang TW, et al. Updated 10-year outcomes of percutaneous radiofrequency ablation as firstline therapy for single hepatocellular carcinoma <3 cm: emphasis on association of local tumor progression and overall survival. Eur Radiol. 2020; 30:2391–2400.
Article26. Ni JY, Liu SS, Xu LF, Sun HL, Chen YT. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2013; 19:3872–3882.
Article27. Wang X, Hu Y, Ren M, Lu X, Lu G, He S. Efficacy and safety of radiofrequency ablation combined with transcatheter arterial chemoembolization for hepatocellular carcinomas compared with radiofrequency ablation alone: a time-to-event meta-analysis. Korean J Radiol. 2016; 17:93–102.
Article28. Lee S, Kim SH, Lee JE, Sinn DH, Park CK. Preoperative gadoxetic acidenhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J Hepatol. 2017; 67:526–534.29. Choi SH, Lee SS, Park SH, Kim KM, Yu E, Park Y, et al. LI-RADS classification and prognosis of primary liver cancers at gadoxetic acid-enhanced MRI. Radiology. 2019; 290:388–397.
Article30. Rhim H, Choi D, Kim YS, Lim HK, Choe BK. Ultrasonography-guided percutaneous radiofrequency ablation of hepatocellular carcinomas: a feasibility scoring system for planning sonography. Eur J Radiol. 2010; 75:253–258.
Article31. Lee MW, Rhim H, Cha DI, Kim YJ, Lim HK. Planning US for percutaneous radiofrequency ablation of small hepatocellular carcinomas (1-3 cm): value of fusion imaging with conventional US and CT/MR images. J Vasc Interv Radiol. 2013; 24:958–965.
Article32. Lee MW, Lim HK, Rhim H, Cha DI, Kang TW, Song KD, et al. Percutaneous radiofrequency ablation of small (1-2cm) hepatocellular carcinomas inconspicuous on B-mode ultrasonographic imaging: usefulness of combined fusion imaging with MRI and contrast-enhanced ultrasonography. Can J Gastroenterol Hepatol. 2018; 2018:7926923.
Article33. Bhatia SS, Spector S, Echenique A, Froud T, Suthar R, Lawson I, et al. Is antibiotic prophylaxis for percutaneous radiofrequency ablation (RFA) of primary liver tumors necessary? results from a single-center experience. Cardiovasc Intervent Radiol. 2015; 38:922–928.34. Chehab MA, Thakor AS, Tulin-Silver S, Connolly BL, Cahill AM, Ward TJ, et al. Adult and pediatric antibiotic prophylaxis during vascular and IR procedures: a Society of Interventional Radiology practice parameter update endorsed by the Cardiovascular and Interventional Radiological Society of Europe and the Canadian Association for Interventional Radiology. J Vasc Interv Radiol. 2018; 29:1483–1501.e2.
Article35. Chen PY, Tsai TJ, Yang HY, Chou CK, Chang LJ, Chen TH, et al. The incidence of bacteremia and risk factors of post-radiofrequency ablation fever for patients with hepato-cellular carcinoma. Cancers (Basel). 2021; 13:5303.
Article36. Hoffmann R, Rempp H, Schmidt D, Pereira PL, Claussen CD, Clasen S. Prolonged antibiotic prophylaxis in patients with bilioenteric anastomosis undergoing percutaneous radiofrequency ablation. J Vasc Interv Radiol. 2012; 23:545–551.
Article37. Lim H, Gong EJ, Min BH, Kang SJ, Shin CM, Byeon JS, et al. Clinical practice guideline for the management of antithrombotic agents in patients undergoing gastrointestinal endoscopy. Clin Endosc. 2020; 53:663–677.
Article38. Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology. 2009; 49:1017–1044.39. Ahn SJ, Lee JM, Lee DH, Lee SM, Yoon JH, Kim YJ, et al. Real-time US-CT/MR fusion imaging for percutaneous radiofrequency ablation of hepatocellular carcinoma. J Hepatol. 2017; 66:347–354.
Article40. Lee MW, Rhim H, Cha DI, Kim YJ, Choi D, Kim YS, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma: fusion imaging guidance for management of lesions with poor conspicuity at conventional sonography. AJR Am J Roentgenol. 2012; 198:1438–1444.
Article41. Park HJ, Lee MW, Lee MH, Hwang J, Kang TW, Lim S, et al. Fusion imaging-guided percutaneous biopsy of focal hepatic lesions with poor conspicuity on conventional sonography. J Ultrasound Med. 2013; 32:1557–1564.
Article42. Song KD, Lee MW, Rhim H, Cha DI, Chong Y, Lim HK. Fusion imagingguided radiofrequency ablation for hepatocellular carcinomas not visible on conventional ultrasound. AJR Am J Roentgenol. 2013; 201:1141–1147.
Article43. Huang JX, Shi CG, Xu YF, Fu J, Zhong Y, Liu LZ, et al. The benefit of contrast-enhanced ultrasound in biopsies for focal liver lesions: a retrospective study of 820 cases. Eur Radiol. 2022; 32:6830–6839.44. Wu W, Jing X, Xue GQ, Zhu XL, Wang J, Du RQ, et al. A multicenter randomized controlled study of contrast-enhanced US versus US-guided biopsy of focal liver lesions. Radiology. 2022; 305:721–728.
Article45. Masuzaki R, Shiina S, Tateishi R, Yoshida H, Goto E, Sugioka Y, et al. Utility of contrast-enhanced ultrasonography with Sonazoid in radiofrequency ablation for hepatocellular carcinoma. J Gastroenterol Hepatol. 2011; 26:759–764.
Article46. Dohmen T, Kataoka E, Yamada I, Miura K, Ohshima S, Shibuya T, et al. Efficacy of contrast-enhanced ultrasonography in radiofrequency ablation for hepatocellular carcinoma. Intern Med. 2012; 51:1–7.
Article47. Lee JY, Minami Y, Choi BI, Lee WJ, Chou YH, Jeong WK, et al. The AFSUMB consensus statements and recommendations for the clinical practice of contrast-enhanced ultrasound using sonazoid. Ultrasonography. 2020; 39:191–220.
Article48. Yanagisawa K, Moriyasu F, Miyahara T, Yuki M, Iijima H. Phagocytosis of ultrasound contrast agent microbubbles by Kupffer cells. Ultrasound Med Biol. 2007; 33:318–325.49. Huang Q, Li J, Zeng Q, Tan L, Zheng R, He X, et al. Value of artificial ascites to assist thermal ablation of liver cancer adjacent to the gastrointestinal tract in patients with previous abdominal surgery. BMC Cancer. 2020; 20:763.
Article50. Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation with artificial ascites for hepatocellular carcinoma in the hepatic dome: initial experience. AJR Am J Roentgenol. 2008; 190:91–98.
Article51. Park SJ, Lee DH, Han JK. Reducing pain by artificial ascites infusion during radiofrequency ablation for subcapsular hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2021; 44:565–573.
Article52. Koda M, Ueki M, Maeda Y, Mimura K, Okamoto K, Matsunaga Y, et al. Percutaneous sonographically guided radiofrequency ablation with artificial pleural effusion for hepatocellular carcinoma located under the diaphragm. AJR Am J Roentgenol. 2004; 183:583–588.
Article53. Nakamura S, Nouso K, Onishi H, Kuwaki K, Hagihara H, Takeuchi Y, et al. Prevention of vagotonia and pain during radiofrequency ablation of liver tumors. Hepatol Res. 2014; 44:1367–1370.54. Yokoyama K, Ikeda O, Kawanaka K, Nakasone Y, Inoue S, Tamura Y, et al. Pain control in patients with hepatocellular carcinoma treated by percutaneous radiofrequency ablation: comparison of the efficacy of one-shot and continuous intravenous fentanyl delivery. Acta Radiol. 2014; 55:1219–1225.
Article55. Dou Z, Lu F, Ren L, Song X, Li B, Li X. Efficacy and safety of microwave ablation and radiofrequency ablation in the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Medicine (Baltimore). 2022; 101:e29321.
Article56. Wang C, Wang H, Yang W, Hu K, Xie H, Hu KQ, et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology. 2015; 61:1579–1590.
Article57. Huang GL, Liu M, Zhang XE, Liu BX, Xu M, Lin MX, et al. Multiple-electrode switching-based radiofrequency ablation vs. conventional radiofrequency ablation for single early-stage hepatocellular carcinoma ranging from 2 to 5 cm. Front Oncol. 2020; 10:1150.
Article58. Lee J, Lee JM, Yoon JH, Lee JY, Kim SH, Lee JE, et al. Percutaneous radiofrequency ablation with multiple electrodes for medium-sized hepatocellular carcinomas. Korean J Radiol. 2012; 13:34–43.59. Laeseke PF, Frey TM, Brace CL, Sampson LA, Winter TC 3rd, Ketzler JR, et al. Multiple-electrode radiofrequency ablation of hepatic malignancies: initial clinical experience. AJR Am J Roentgenol. 2007; 188:1485–1494.
Article60. Suh YS, Choi JW, Yoon JH, Lee DH, Kim YJ, Lee JH, et al. No-touch vs. conventional radiofrequency ablation using twin internally cooled wet electrodes for small hepatocellular carcinomas: a randomized prospective comparative study. Korean J Radiol. 2021; 22:1974–1984.
Article61. Park SJ, Cho EJ, Lee JH, Yu SJ, Kim YJ, Yoon JH, et al. Switching monopolar no-touch radiofrequency ablation using octopus electrodes for small hepatocellular carcinoma: a randomized clinical trial. Liver Cancer. 2021; 10:72–81.
Article62. Lee DH, Lee MW, Kim PN, Lee YJ, Park HS, Lee JM. Outcome of notouch radiofrequency ablation for small hepatocellular carcinoma: a multicenter clinical trial. Radiology. 2021; 301:229–236.
Article63. Ei S, Hibi T, Tanabe M, Itano O, Shinoda M, Kitago M, et al. Cryoablation provides superior local control of primary hepatocellular carcinomas of >2 cm compared with radiofrequency ablation and microwave coagulation therapy: an underestimated tool in the toolbox. Ann Surg Oncol. 2015; 22:1294–1300.64. Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology. 2014; 273:241–260.
Article65. Crocetti L, de Baere T, Lencioni R. Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc Intervent Radiol. 2010; 33:11–17.
Article66. Dodd GD 3rd, Napier D, Schoolfield JD, Hubbard L. Percutaneous radiofrequency ablation of hepatic tumors: postablation syndrome. AJR Am J Roentgenol. 2005; 185:51–57.
Article67. Carrafiello G, Laganà D, Ianniello A, Dionigi G, Novario R, Recaldini C, et al. Post-radiofrequency ablation syndrome after percutaneous radiofrequency of abdominal tumours: one centre experience and review of published works. Australas Radiol. 2007; 51:550–554.
Article68. Kim JW, Shin JH, Kim PN, Shin YM, Won HJ, Ko GY, et al. Embolization for bleeding after hepatic radiofrequency ablation. J Vasc Interv Radiol. 2017; 28:356–365.e2.69. Chang IS, Kim YJ, Park SW, Park HS, Jeon HJ, Chang SH, et al. Delayed hepatic rupture after radiofrequency ablation for colorectal hepatic metastasis: management with transcatheter arterial embolization. Ann Surg Treat Res. 2014; 87:41–43.
Article70. Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2008; 47:82–89.
Article71. Tan W, Deng Q, Lin S, Wang Y, Xu G. Comparison of microwave ablation and radiofrequency ablation for hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2019; 36:264–272.
Article72. Kim R, Kang TW, Cha DI, Song KD, Lee MW, Rhim H, et al. Percutaneous cryoablation for perivascular hepatocellular carcinoma: therapeutic efficacy and vascular complications. Eur Radiol. 2019; 29:654–662.
Article73. Ko SE, Lee MW, Rhim H, Kang TW, Song KD, Cha DI, et al. Comparison of procedure-related complications between percutaneous cryoablation and radiofrequency ablation for treating periductal hepatocellular carcinoma. Int J Hyperthermia. 2020; 37:1354–1361.74. Song KD. Percutaneous cryoablation for hepatocellular carcinoma. Clin Mol Hepatol. 2016; 22:509–515.
Article75. Choi D, Lim HK, Kim MJ, Kim SJ, Kim SH, Lee WJ, et al. Liver abscess after percutaneous radiofrequency ablation for hepatocellular carcinomas: frequency and risk factors. AJR Am J Roentgenol. 2005; 184:1860–1867.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Local Ablation for Hepatocellular Carcinoma: 2024 Expert Consensus-Based Practical Recommendations of the Korean Liver Cancer Association
- Local Ablation for Hepatocellular Carcinoma: 2024 Expert Consensus-Based Practical Recommendations of the Korean Liver Cancer Association
- Practical consensus multi-specialty guidelines on image-guided ablation for hepatocellular carcinoma
- Radiofrequency Ablation for Hepatocellular Carcinoma
- 2018 Korean Liver Cancer Association–National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma