Ann Pediatr Endocrinol Metab.
2024 Aug;29(4):266-275. 10.6065/apem.2346100.050.
Glycated albumin may have a complementary role to glycated hemoglobin in glucose monitoring in childhood acute leukemia
- Affiliations
-
- 1Department of Pediatrics, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2559455
- DOI: http://doi.org/10.6065/apem.2346100.050
Abstract
- Purpose
Glycated hemoglobin (HbA1c) as a glycemic index may have limited value in pediatric patients with acute leukemia as they often present with anemia and/or pancytopenia. To address this issue, we evaluated the usefulness of glycated albumin (GA) as a glycemic monitoring index in pediatric patients with acute leukemia.
Methods
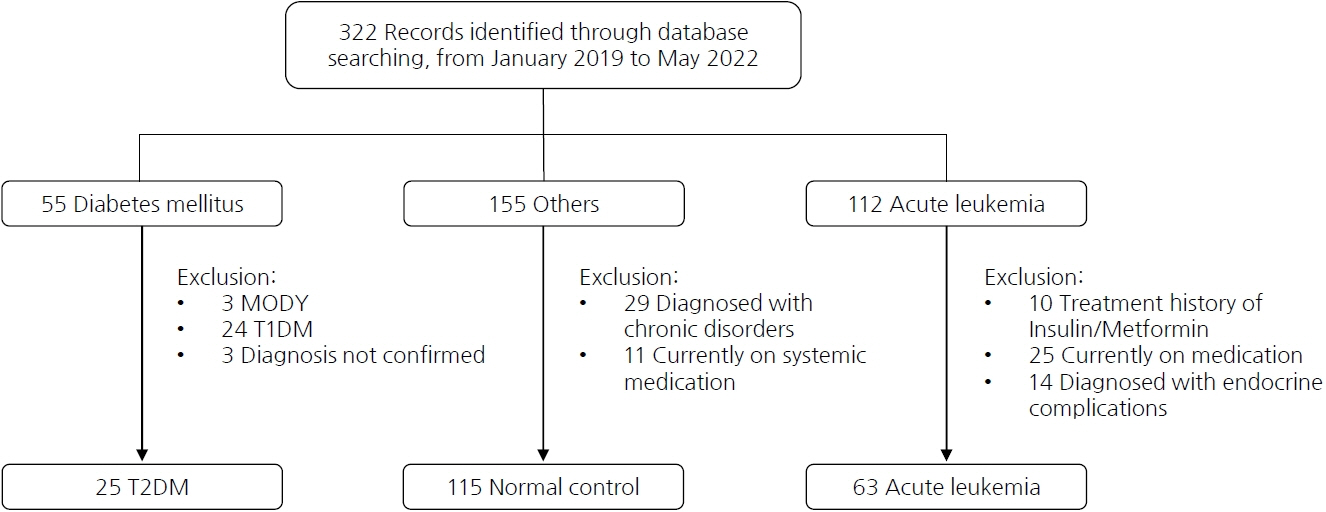
Medical records of 25 patients with type 2 diabetes mellitus (T2DM), 63 patients with acute leukemia, and 115 healthy children from Seoul St. Mary's Hospital, The Catholic University of Korea, were retrospectively investigated for serum GA, HbA1c, and fasting blood glucose (FBG) levels, along with demographic data.
Results
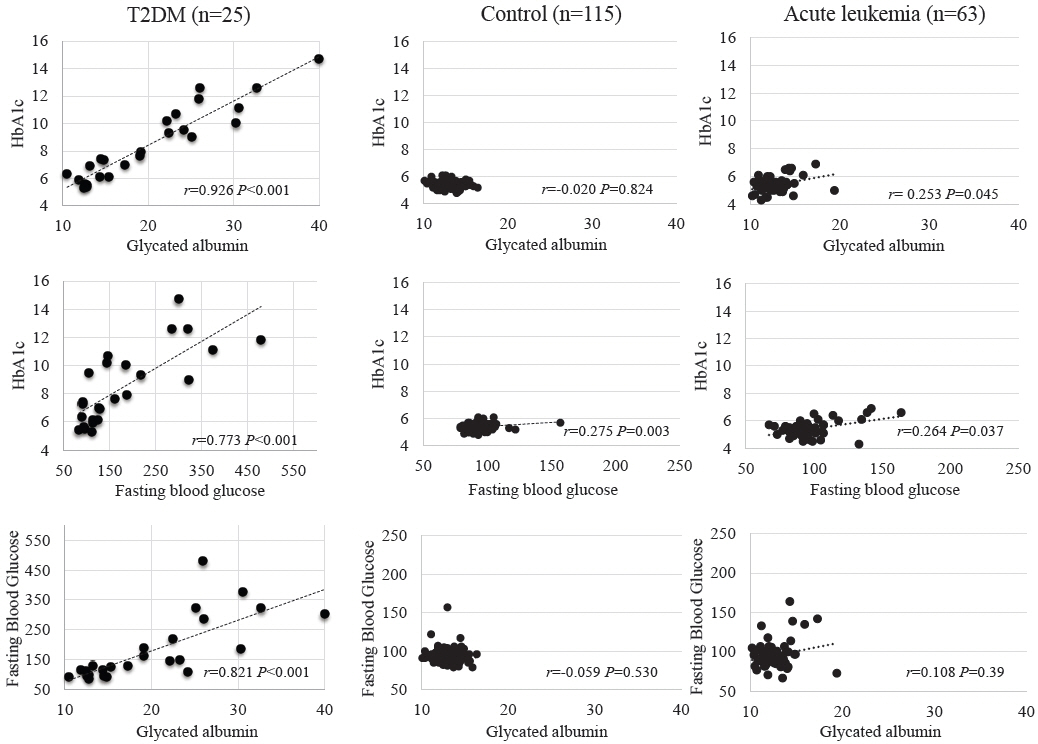
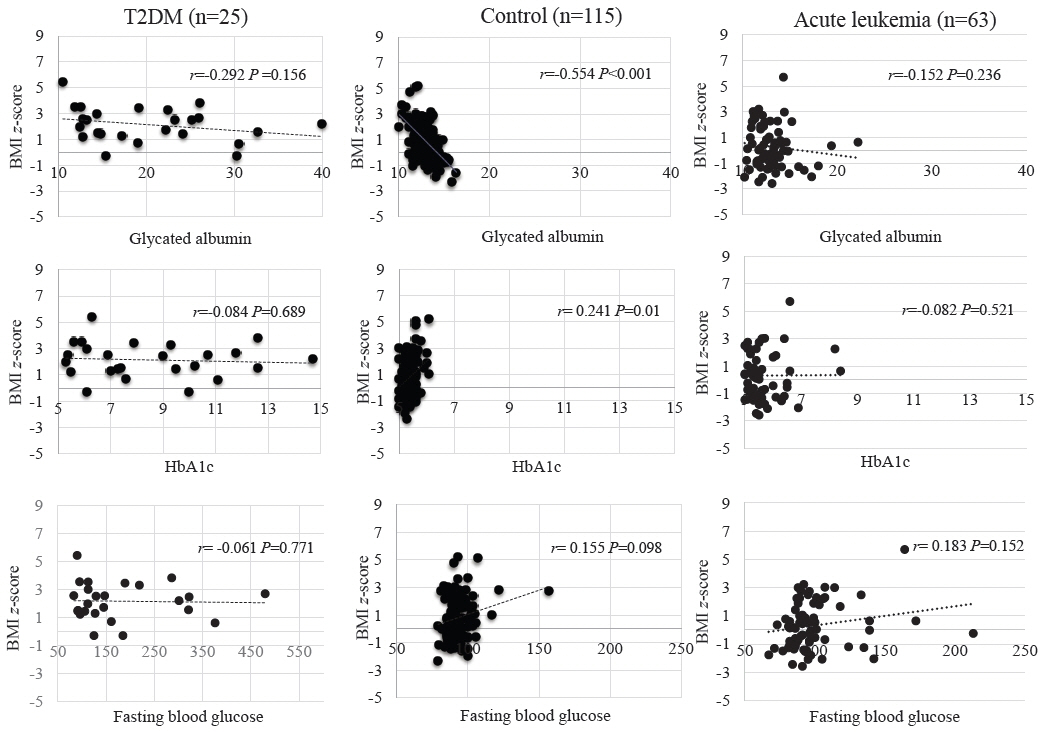
GA, HbA1c, and FBG levels did not differ between the control and acute leukemia groups. In the T2DM group, positive correlations were observed among GA, HbA1c, and FBG (P<0.01). Although GA level was not associated with the HbA1c level in the control group, GA and HbA1c levels showed a positive correlation in the acute leukemia group (P=0.045). Regression analysis revealed GA and HbA1c levels to be positively correlated in the acute leukemia and T2DM groups even after adjusting for age, sex, and body mass index z-score (P=0.007, P<0.01).
Conclusion
GA may be a useful complementary parameter to HbA1c for glycemic monitoring in pediatric patients with acute leukemia, similar to its use in patients with T2DM.
Figure
Reference
-
References
1. Kononczuk K, Muszynska-Roslan K, Konstantynowicz-Nowicka K, Krawczuk-Rybak M, Chabowski A, Latoch E. Biomarkers of glucose metabolism alterations and the onset of metabolic syndrome in survivors of childhood acute lymphoblastic leukemia. Int J Mol Sci. 2022; 23:3712.
Article2. Bahoush G, Salajegheh P, Rohani F. Association between body mass index and insulin resistance in survivors of pediatric acute lymphoblastic leukemia. Leuk Res Rep. 2020; 13:100199.
Article3. Pluimakers VG, van Waas M, Neggers S, van den Heuvel-Eibrink MM. Metabolic syndrome as cardiovascular risk factor in childhood cancer survivors. Crit Rev Oncol Hematol. 2019; 133:129–41.
Article4. Sonabend RY, McKay SV, Okcu MF, Yan J, Haymond MW, Margolin JF. Hyperglycemia during induction therapy is associated with poorer survival in children with acute lymphocytic leukemia. J Pediatr. 2009; 155:73–8.
Article5. Gregoriou K, Craigie I, Gibson B, Mason A, Shaikh MG. Risk factors and management of corticosteroid-induced hyperglycaemia in paediatric acute lymphoblastic leukaemia. Pediatr Blood Cancer. 2020; 67:e28085.6. Handattu K, Sharma LK, Vijayasekharan K, Bhat KV, Aroor S, Sudhanshu S. Drug induced diabetes mellitus in pediatric acute lymphoblastic leukemia: approach to diagnosis and management. J Pediatr Hematol Oncol. 2022; 44:273–9.
Article7. Williams HE, Howell CR, Chemaitilly W, Wilson CL, Karol SE, Nolan VG, et al. Diabetes mellitus among adult survivors of childhood acute lymphoblastic leukemia: a report from the St. Jude Lifetime Cohort Study. Cancer. 2020; 126:870–8.
Article8. Chan CL, Pyle L, Kelsey M, Newnes L, Zeitler PS, Nadeau KJ. Screening for type 2 diabetes and prediabetes in obese youth: evaluating alternate markers of glycemia - 1,5-anhydroglucitol, fructosamine, and glycated albumin. Pediatr Diabetes. 2016; 17:206–11.
Article9. Spencer DH, Grossman BJ, Scott MG. Red cell transfusion decreases hemoglobin A1c in patients with diabetes. Clin Chem. 2011; 57:344–6.
Article10. Wei C, Unsworth R, Davis N, Cox R, Bradley K, Stevens M, et al. Survivors of childhood leukaemia treated with haematopoietic stem cell transplantation and total body irradiation should undergo screening for diabetes by oral glucose tolerance tests. Diabet Med. 2016; 33:1347–51.
Article11. Chan CL, McFann K, Newnes L, Nadeau KJ, Zeitler PS, Kelsey M. Hemoglobin A1c assay variations and implications for diabetes screening in obese youth. Pediatr Diabetes. 2014; 15:557–63.
Article12. Musha I, Mochizuki M, Kikuchi T, Akatsuka J, Ohtake A, Kobayashi K, et al. Estimation of glycaemic control in the past month using ratio of glycated albumin to HbA1c. Diabet Med. 2018; 35:855–61.13. American Diabetes A. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care. 2019; 42(Suppl 1):S13–28.14. Kim JH, Yun S, Hwang SS, Shim JO, Chae HW, Lee YJ, et al. The 2017 Korean National Growth Charts for children and adolescents: development, improvement, and prospects. Korean J Pediatr. 2018; 61:135–49.
Article15. Zemlin AE, Barkhuizen M, Kengne AP, Erasmus RT, Matsha TE. Performance of glycated albumin for type 2 diabetes and prediabetes diagnosis in a South African population. Clin Chim Acta. 2019; 488:122–8.
Article16. Hashimoto K, Osugi T, Noguchi S, Morimoto Y, Wasada K, Imai S, et al. A1C but not serum glycated albumin is elevated because of iron deficiency in late pregnancy in diabetic women. Diabetes Care. 2010; 33:509–11.
Article17. Sugawara D, Sato H, Makita E, Kuwata T, Takagi K, Ichihashi K. Clinical usefulness of glycated albumin and glycated albumin-to-glycated hemoglobin ratio of gestational diabetes mellitus in late pregnancy for predicting infant complications. Pediatr Neonatol. 2022; 63:239–46.
Article18. Kim Y, Lee SH, Kang MK, Kim TJ, Jeong HY, Lee EJ, et al. Glycated albumin, a novel biomarker for short-term functional outcomes in acute ischemic stroke. Brain Sci. 2021; 11:337.
Article19. Vos FE, Schollum JB, Walker RJ. Glycated albumin is the preferred marker for assessing glycaemic control in advanced chronic kidney disease. NDT Plus. 2011; 4:368–75.
Article20. Rescalli A, Varoni EM, Cellesi F, Cerveri P. Analytical challenges in diabetes management: towards glycated albumin point-of-care detection. Biosensors (Basel). 2022; 12:687.21. Desouza CV, Holcomb RG, Rosenstock J, Frias JP, Hsia SH, Klein EJ, et al. Results of a study comparing glycated albumin to other glycemic indices. J Clin Endocrinol Metab. 2020; 105:677–87.
Article22. Nishimura R, Kanda A, Sano H, Matsudaira T, Miyashita Y, Morimoto A, et al. Glycated albumin is low in obese, non-diabetic children. Diabetes Res Clin Pract. 2006; 71:334–8.
Article23. Wallace AS, Rooney MR, Brady TM, Echouffo-Tcheugui JB, Christenson R, Grams ME, et al. The performance of glycated albumin as a biomarker of hyperglycemia and cardiometabolic risk in children and adolescents in the United States. Pediatr Diabetes. 2022; 23:237–47.
Article24. Ribeiro RT, Macedo MP, Raposo JF. HbA1c, Fructosamine, and glycated albumin in the detection of dysglycaemic conditions. Curr Diabetes Rev. 2016; 12:14–9.
Article25. Pollock NI, Flamand Y, Zhu J, Millington K, Stevenson K, Silverman LB, et al. Hyperglycemia during induction therapy for acute lymphoblastic leukemia is temporally linked to pegaspargase administration. Pediatr Blood Cancer. 2022; 69:e29505.
Article26. Chueh HW, Yoo JH. Metabolic syndrome induced by anticancer treatment in childhood cancer survivors. Ann Pediatr Endocrinol Metab. 2017; 22:82–9.
Article27. Kartal I, Alacam A, Dagdemir A, Kara C, Dincer OS, Albayrak C, et al. Frequency of obesity and metabolic syndrome in childhood leukemia and lymphoma survivors. Diabetol Metab Syndr. 2022; 14:16.
Article28. Suwa T, Ohta A, Matsui T, Koganei R, Kato H, Kawata T, et al. Relationship between clinical markers of glycemia and glucose excursion evaluated by continuous glucose monitoring (CGM). Endocr J. 2010; 57:135–40.
Article29. McCormick MC, Sharp E, Kalpatthi R, Zullo J, Gurtunca N, Zhang J, et al. Hyperglycemia requiring insulin during acute lymphoblastic leukemia induction chemotherapy is associated with increased adverse outcomes and healthcare costs. Pediatr Blood Cancer. 2020; 67:e28475.
Article30. Saultier P, Auquier P, Bertrand Y, Vercasson C, Oudin C, Contet A, et al. Metabolic syndrome in long-term survivors of childhood acute leukemia treated without hematopoietic stem cell transplantation: an L.E.A. study. Haematologica. 2016; 101:1603–10.
Article31. Brignardello E, Felicetti F, Castiglione A, Chiabotto P, Corrias A, Fagioli F, et al. Endocrine health conditions in adult survivors of childhood cancer: the need for specialized adult-focused follow-up clinics. Eur J Endocrinol. 2013; 168:465–72.
Article32. Piona C, Marigliano M, Mozzillo E, Rosanio F, Zanfardino A, Iafusco D, et al. Relationships between HbA1c and continuous glucose monitoring metrics of glycaemic control and glucose variability in a large cohort of children and adolescents with type 1 diabetes. Diabetes Res Clin Pract. 2021; 177:108933.
Article33. Desouza CV, Rosenstock J, Zhou R, Holcomb RG, Fonseca VA. Glycated albumin at 4 weeks correlates with a1c levels at 12 weeks and reflects short-term glucose fluctuations. Endocr Pract. 2015; 21:1195–203.
Article34. Sugimoto T, Hashimoto M, Hayakawa I, Tokuno O, Ogino T, Okuno M, et al. Alterations in HbA1c resulting from the donation of autologous blood for elective surgery in patients with diabetes mellitus. Blood Transfus. 2014; 12 Suppl 1(Suppl 1):s209–13.35. Janice D, Prathima MB, Sushith S, Narayanan R, Reshma S, Nair S, et al. Effect of iron deficiency anaemia over glycated hemoglobin in non-diabetic women. Int J Biochem Mol Biol. 2022; 13:23–7.36. Koga M, Otsuki M, Matsumoto S, Saito H, Mukai M, Kasayama S. Negative association of obesity and its related chronic inflammation with serum glycated albumin but not glycated hemoglobin levels. Clin Chim Acta. 2007; 378:48–52.
Article37. Kim M, Kim J. Cardiometabolic risk factors and metabolic syndrome based on severity of obesity in Korean children and adolescents: data from the Korea National Health and Nutrition Examination Survey 2007-2018. Ann Pediatr Endocrinol Metab. 2022; 27:289–99.
Article38. Kim JH, Lim JS. The association between C-reactive protein, metabolic syndrome, and prediabetes in Korean children and adolescents. Ann Pediatr Endocrinol Metab. 2022; 27:273–80.
Article39. Park HK, Seo JY, Jung HW, Lim JS. Prevalence and trends in obesity and severe obesity in Korean children and adolescents, 2007-2020: a population-based study. Pediatr Int. 2023; 65:e15472.
Article40. Kang HM, Jeong DC, Suh BK, Ahn MB. The impact of the coronavirus disease-2019 pandemic on childhood obesity and vitamin D status. J Korean Med Sci. 2021; 36:e21.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Alternative biomarkers for assessing glycemic control in diabetes: fructosamine, glycated albumin, and 1,5-anhydroglucitol
- Response: Predictors of the Therapeutic Efficacy and Consideration of the Best Combination Therapy of Sodium-Glucose Co-transporter 2 Inhibitors (Diabetes Metab J 2019;43:158–73)
- Letter: Predictors of the Therapeutic Efficacy and Consideration of the Best Combination Therapy of Sodium-Glucose Co-transporter 2 Inhibitors (Diabetes Metab J 2019;43:158–73)
- How Do We Diagnose Diabetes in Primary Care?
- Review of the Potential Glycemic Markers Glycated Albumin and 1,5-anhydroglucitol