Kosin Med J.
2024 Sep;39(3):186-194. 10.7180/kmj.24.103.
Preliminary data on computed tomography-based radiomics for predicting programmed death ligand 1 expression in urothelial carcinoma
- Affiliations
-
- 1Department of Radiology, Biomedical Research Institute, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea
- 2Department of Urology, Biomedical Research Institute, Pusan National University Hospital , Pusan National University School of Medicine, Busan, Korea
- 3Department of Urology, Dongnam Institute of Radiological and Medical Sciences Cancer Center, Busan, Korea
- KMID: 2559406
- DOI: http://doi.org/10.7180/kmj.24.103
Abstract
- Background
Programmed death ligand 1 (PD-L1) expression cannot currently be predicted through radiological findings. This study aimed to develop a prediction model capable of differentiating between positive and negative PD-L1 expression through a radiomics-based investigation of computed tomography (CT) images in patients with urothelial carcinoma.
Methods
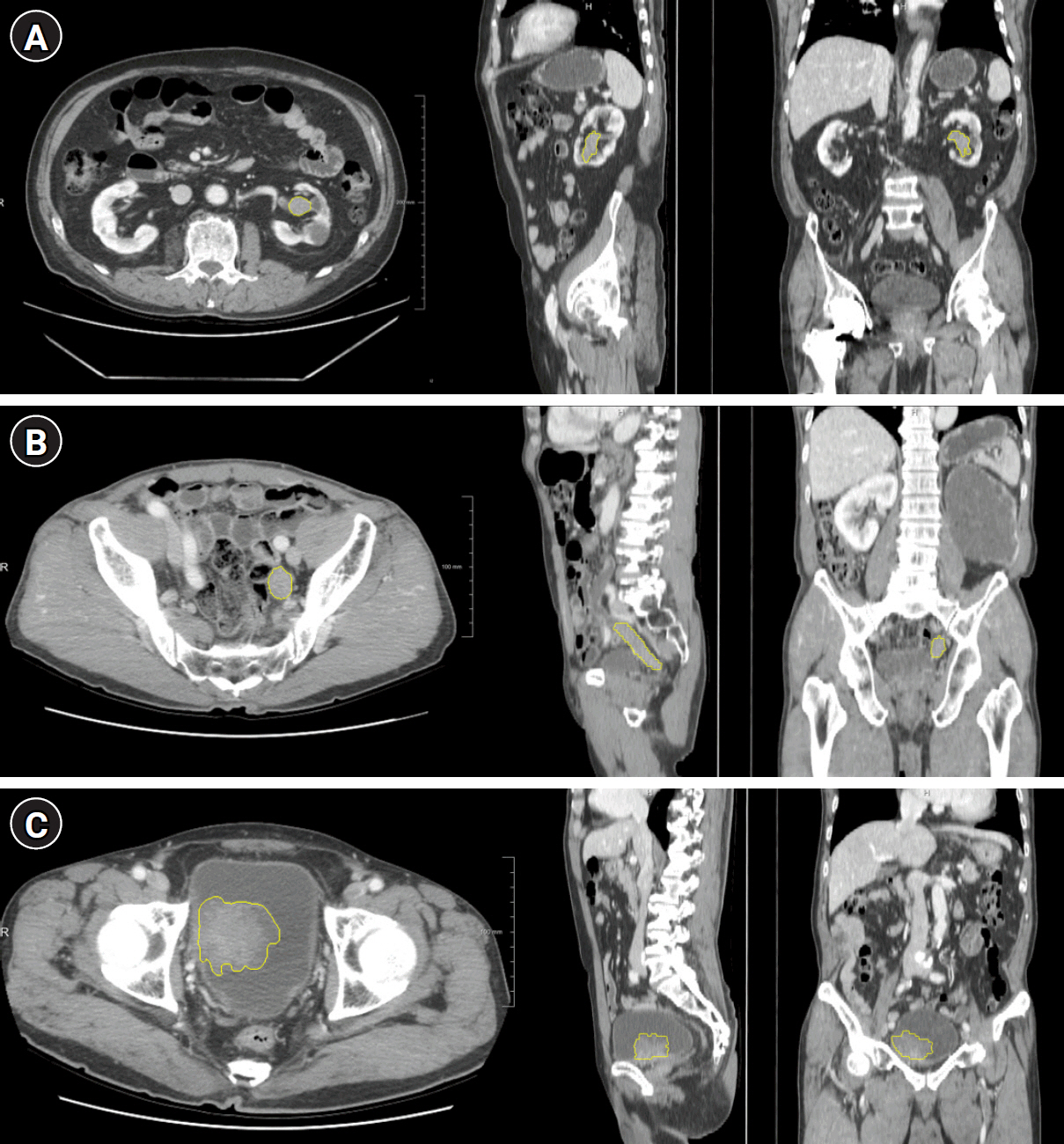
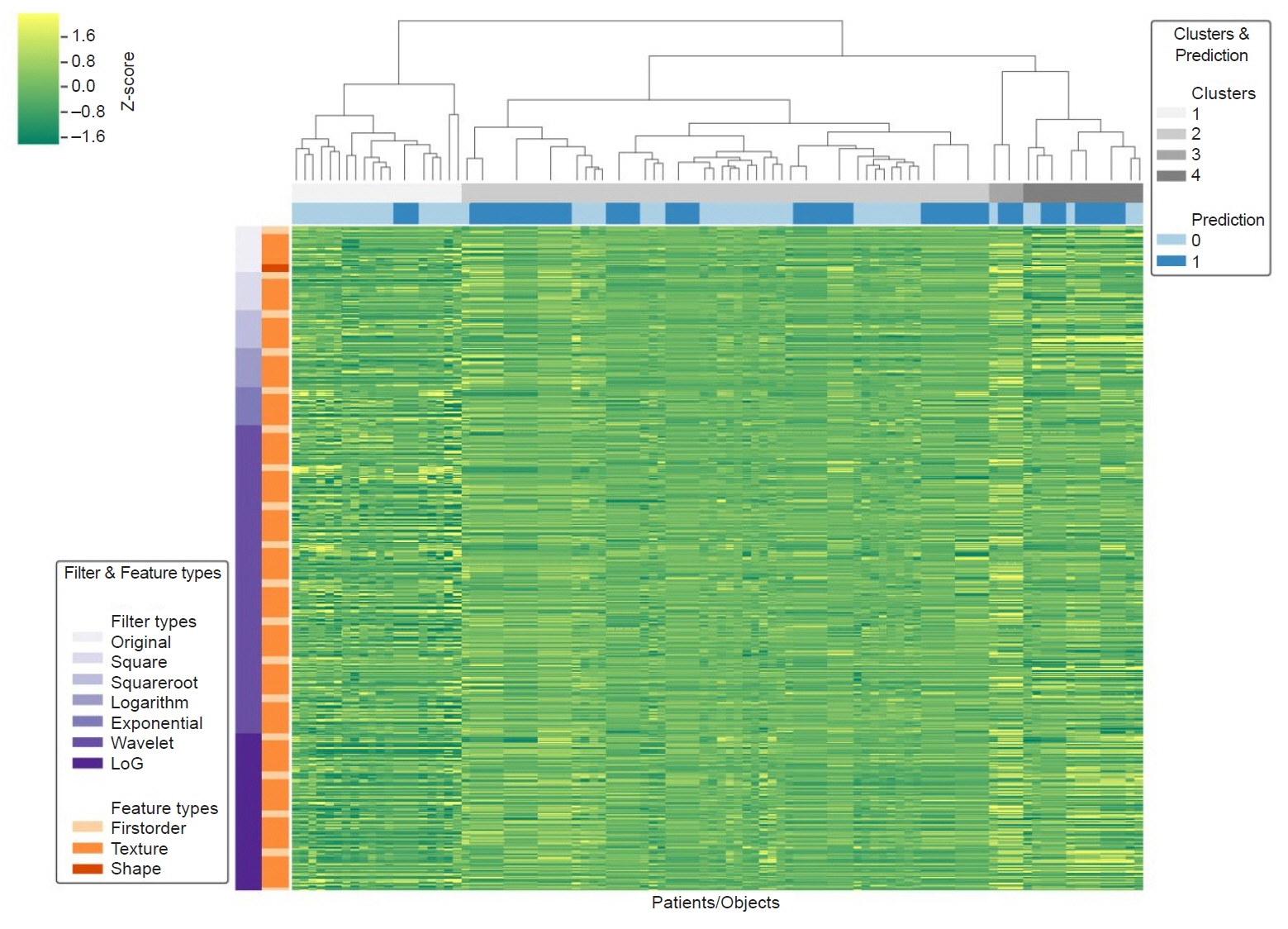
Sixty-four patients with urothelial carcinoma who underwent immunohistochemical testing for PD-L1 were retrospectively reviewed. The number of patients in the positive and negative PD-L1 groups (PD-L1 expression >5%) was 14 and 50, respectively. CT images obtained 90 seconds after contrast medium administration were selected for radiomic extraction. For all tumors, 1,691 radiomic features were extracted from CT using a manually segmented three-dimensional volume of interest. Univariate and multivariate logistic regression analyses were performed to identify radiomic features that were significant predictors of PD-L1 expression. For the radiomics-based model, a receiver operating characteristic (ROC) analysis was performed.
Results
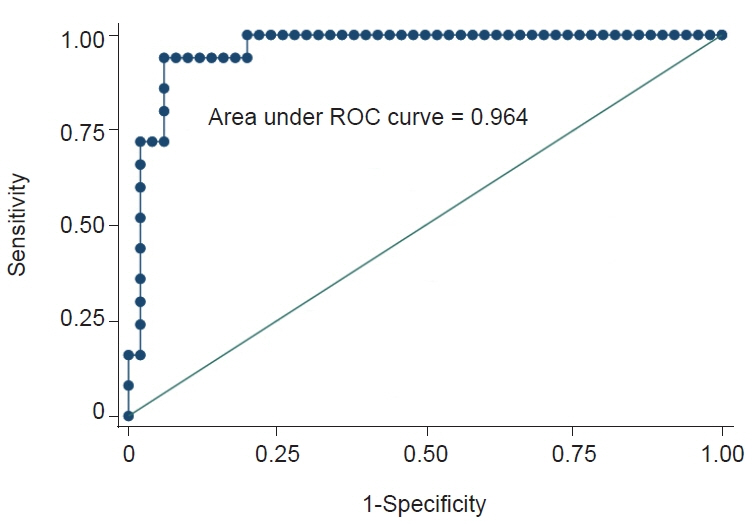
Among 64 patients, 14 were included in the PD-L1 positive group. Logistic regression analysis found that the following radiomic features significantly predicted PD-L1 expression: wavelet-low-pass, low-pass, and high-pass filters (LLH)_gray-level size-zone matrix (GLSZM)_SmallAreaEmphasis, wavelet-LLH_firstorder_Energy, log-sigma-0-5-mm-3D_GLSZM_SmallAreaHighGrayLevelEmphasis, original_shape_Maximum2DDiameterColumn, wavelet-low-pass, low-pass, and low-pass filters (LLL)_gray-level run-length matrix (GLRLM)_ShortRunEmphasis, and exponential_firstorder_Kurtosis. The radiomics signature was –4.0934+21.6224 (wavelet-LLH_GLSZM_SmallAreaEmphasis)+0.0044 (wavelet-LLH_firstorder_Energy)–4.7389 (log-sigma-0-5-mm-3D_GLSZM_SmallAreaHighGrayLevelEmphasis)+0.0573 (original_shape_Maximum2DDiameterColumn)–29.5892 (wavelet-LLL_GLRLM_ShortRunEmphasis)–0.4324 (exponential_firstorder_Kurtosis). The area under the ROC curve model representing the radiomics signature for differentiating cases that were deemed PD-L1 positive based on immunohistochemistry was 0.96.
Conclusions
This preliminary radiomics model derived from contrast-enhanced CT predicted PD-L1 positivity in patients with urothelial cancer.
Figure
Reference
-
References
1. Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017; 71:96–108.
Article2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.3. Seront E, Machiels JP. Molecular biology and targeted therapies for urothelial carcinoma. Cancer Treat Rev. 2015; 41:341–53.
Article4. Oing C, Rink M, Oechsle K, Seidel C, von Amsberg G, Bokemeyer C. Second line chemotherapy for advanced and metastatic urothelial carcinoma: vinflunine and beyond: a comprehensive review of the current literature. J Urol. 2016; 195:254–63.5. Rouanne M, Loriot Y, Lebret T, Soria JC. Novel therapeutic targets in advanced urothelial carcinoma. Crit Rev Oncol Hematol. 2016; 98:106–15.
Article6. Balar AV, Castellano D, O’Donnell PH, Grivas P, Vuky J, Powles T, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017; 18:1483–92.
Article7. Davarpanah NN, Yuno A, Trepel JB, Apolo AB. Immunotherapy: a new treatment paradigm in bladder cancer. Curr Opin Oncol. 2017; 29:184–95.8. Bidnur S, Savdie R, Black PC. Inhibiting immune checkpoints for the treatment of bladder cancer. Bladder Cancer. 2016; 2:15–25.
Article9. Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017; 376:1015–26.
Article10. Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016; 387:1909–20.
Article11. Loriot Y, Rosenberg JE, Powles TB, Necchi A, Hussain S, Morales R, et al. Atezolizumab (atezo) in platinum (plat)-treated locally advanced/metastatic urothelial carcinoma (mUC): updated OS, safety and biomarkers from the Ph II IMvigor210 study. Ann Oncol. 2016; 27:vi270.
Article12. Gillies RJ, Anderson AR, Gatenby RA, Morse DL. The biology underlying molecular imaging in oncology: from genome to anatome and back again. Clin Radiol. 2010; 65:517–21.13. Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014; 5:4006.
Article14. Bak SH, Park H, Lee HY, Kim Y, Kim HL, Jung SH, et al. Imaging genotyping of functional signaling pathways in lung squamous cell carcinoma using a radiomics approach. Sci Rep. 2018; 8:3284.
Article15. Huang YQ, Liang CH, He L, Tian J, Liang CS, Chen X, et al. Development and validation of a radiomics nomogram for preoperative prediction of lymph node metastasis in colorectal cancer. J Clin Oncol. 2016; 34:2157–64.
Article16. Xu X, Zhang HL, Liu QP, Sun SW, Zhang J, Zhu FP, et al. Radiomic analysis of contrast-enhanced CT predicts microvascular invasion and outcome in hepatocellular carcinoma. J Hepatol. 2019; 70:1133–44.
Article17. Bellmunt J, Mullane SA, Werner L, Fay AP, Callea M, Leow JJ, et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol. 2015; 26:812–7.18. Sun R, Limkin EJ, Vakalopoulou M, Dercle L, Champiat S, Han SR, et al. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study. Lancet Oncol. 2018; 19:1180–91.
Article19. Nardone V, Tini P, Pastina P, Botta C, Reginelli A, Carbone SF, et al. Radiomics predicts survival of patients with advanced non-small cell lung cancer undergoing PD-1 blockade using Nivolumab. Oncol Lett. 2020; 19:1559–66.
Article20. Yoon J, Suh YJ, Han K, Cho H, Lee HJ, Hur J, et al. Utility of CT radiomics for prediction of PD-L1 expression in advanced lung adenocarcinomas. Thorac Cancer. 2020; 11:993–1004.
Article21. Park KJ, Lee JL, Yoon SK, Heo C, Park BW, Kim JK. Radiomics-based prediction model for outcomes of PD-1/PD-L1 immunotherapy in metastatic urothelial carcinoma. Eur Radiol. 2020; 30:5392–403.
Article22. Pyradiomics Community. Open-source python package for the extraction of Radiomics features from medical imaging [Internet]. Pyradiomics Community;c2016. [cited 2022 Nov 30]. https://pyradiomics.readthedocs.io/en/latest/index.html.23. Shur JD, Doran SJ, Kumar S, Ap Dafydd D, Downey K, O’Connor JP, et al. Radiomics in oncology: a practical guide. Radiographics. 2021; 41:1717–32.24. Hou Z, Yang Y, Li S, Yan J, Ren W, Liu J, et al. Radiomic analysis using contrast-enhanced CT: predict treatment response to pulsed low dose rate radiotherapy in gastric carcinoma with abdominal cavity metastasis. Quant Imaging Med Surg. 2018; 8:410–20.
Article25. Nakanishi J, Wada Y, Matsumoto K, Azuma M, Kikuchi K, Ueda S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother. 2007; 56:1173–82.
Article26. Inman BA, Sebo TJ, Frigola X, Dong H, Bergstralh EJ, Frank I, et al. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007; 109:1499–505.
Article27. Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014; 515:558–62.
Article28. Paquier Z, Chao SL, Acquisto A, Fenton C, Guiot T, Dhont J, et al. Radiomics software comparison using digital phantom and patient data: IBSI-compliance does not guarantee concordance of feature values. Biomed Phys Eng Express. 2022; 8:065008.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sarcomatoid urothelial carcinoma arising in the female urethral diverticulum
- Programmed cell death-ligand 1 assessment in urothelial carcinoma: prospect and limitation
- Exploring the mythical abscopal effect: Radiation and programmed cell death protein 1 (PD-1) blockade for hepatocellular carcinoma
- A Case of Anti-programmed Cell Death Ligand 1 and Anti-transforming Growth Factor Beta Antibody-associated Keratoacanthoma
- Systemic treatment for advanced urothelial cancer: an update on recent clinical trials and current treatment options