J Korean Med Sci.
2024 Aug;39(33):e234. 10.3346/jkms.2024.39.e234.
First-in-Human Evaluation of a Polymer-Free Everolimus-Eluting Stent Using a Titanium Dioxide Film
- Affiliations
-
- 1Department of Cardiovascular Medicine, Chonnam National University Hospital, Chonnam National University School of Medicine, Gwangju, Korea
- 2Cardiovascular Research Center, Chonnam National University Hospital, Gwangju, Korea
- 3Korea Cardiovascular Stent Institute, Jangseong, Korea
- 4CG Bio Co., Ltd., Seoul, Korea
- 5Heart and Brain Hospital, Chung-Ang University Hospital, Chung-Ang University College of Medicine, Seoul, Korea
- 6Gwangju Veterans Hospital, Gwangju, Korea
- KMID: 2559226
- DOI: http://doi.org/10.3346/jkms.2024.39.e234
Abstract
- Background
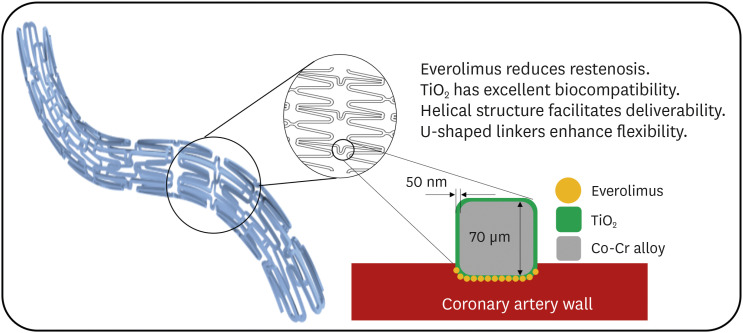
In patients with coronary artery disease treated with permanent polymercoated drug-eluting stents (DES), the persistent presence of a less biocompatible polymer might delay arterial healing. Thin strut polymer-free DES have the potential to improve clinical outcomes and reduce the duration of dual antiplatelet therapy (DAPT). The purpose of this first-in-human study was to assess the safety and effectiveness of a novel polymer-free DES in patients with de novo coronary lesions. The TIGERevolutioN® stent (CG Bio Co., Ltd., Seoul, Korea) consists of a cobalt chromium platform with a strut thickness of 70 μm and a surface treated with titanium dioxide onto which everolimus-eluting stent (EES) is applied abluminally (6 µg/mm of stent length) without utilization of a polymer.
Methods
A total of 20 patients were enrolled, with de novo coronary lesions (stable or unstable angina) and > 50% diameter stenosis in a vessel 2.25 to 4.00 mm in diameter and ≤ 40 mm in length for angiographic, optical coherence tomography (OCT), and clinical assessment at 8 months. All patients received DAPT after stent implantation. The primary endpoint was angiographic in-stent late lumen loss (LLL) at 8 months.
Results
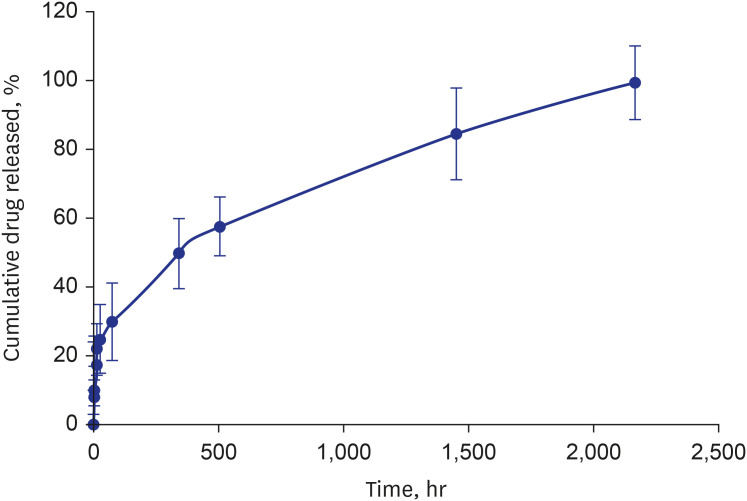
Twenty patients with 20 lesions were treated with TIGERevolutioN® . At 8 months, in-stent LLL was 0.7 ± 0.4 mm. On OCT, percent area stenosis was 29.2 ± 9.4% and stent strut coverage was complete in all lesions. No adverse cardiovascular event occurred at 8 months.
Conclusion
The new polymer-free EES was safe and effective with low LLL and excellent strut coverage at 8 months of follow-up.
Keyword
Figure
Reference
-
1. Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002; 346(23):1773–1780. PMID: 12050336.2. Stone GW, Ellis SG, Cox DA, Hermiller J, O’Shaughnessy C, Mann JT, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004; 350(3):221–231. PMID: 14724301.3. Indolfi C, Pavia M, Angelillo IF. Drug-eluting stents versus bare metal stents in percutaneous coronary interventions (a meta-analysis). Am J Cardiol. 2005; 95(10):1146–1152. PMID: 15877984.4. Kim W, Jeong MH, Hwang SH, Kim KH, Hong YJ, Ahn YK, et al. Comparison of abciximab combined with dalteparin or unfractionated heparin in high-risk percutaneous coronary intervention in acute myocardial infarction patients. Int Heart J. 2006; 47(6):821–831. PMID: 17268117.5. Murphy JG, Schwartz RS, Edwards WD, Camrud AR, Vlietstra RE, Holmes DR Jr. Percutaneous polymeric stents in porcine coronary arteries. Initial experience with polyethylene terephthalate stents. Circulation. 1992; 86(5):1596–1604. PMID: 1423971.6. Nebeker JR, Virmani R, Bennett CL, Hoffman JM, Samore MH, Alvarez J, et al. Hypersensitivity cases associated with drug-eluting coronary stents: a review of available cases from the Research on Adverse Drug Events and Reports (RADAR) project. J Am Coll Cardiol. 2006; 47(1):175–181. PMID: 16386683.7. Stone GW, Moses JW, Ellis SG, Schofer J, Dawkins KD, Morice MC, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007; 356(10):998–1008. PMID: 17296824.8. Urban P, Meredith IT, Abizaid A, Pocock SJ, Carrié D, Naber C, et al. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med. 2015; 373(21):2038–2047. PMID: 26466021.9. Sim DS, Jeong MH, Park DS, Kim JH, Lim KS, Bae IH, et al. A novel polymer-free drug-eluting stent coated with everolimus using nitrogen-doped titanium dioxide film deposition in a porcine coronary restenosis model. Int J Cardiol. 2016; 222:436–440. PMID: 27505330.10. Cho KH, Jeong MH, Park DS, Kim M, Kim J, Park JK, et al. Preclinical evaluation of a novel polymer-free everolimus-eluting stent in a mid-term porcine coronary restenosis model. J Korean Med Sci. 2021; 36(40):e259. PMID: 34664799.11. Song SJ, Park YJ, Park J, Cho MD, Kim JH, Jeong MH, et al. Preparation of a drug-eluting stent using a TiO2 film deposited by plasma enhanced chemical vapour deposition as a drug-combining matrix. J Mater Chem. 2010; 20(23):4792–4801.12. Song SJ, Jung KW, Park YJ, Park J, Cho MD, Jeong MH, et al. Nitrogen-doped TiO2 films as drug-binding matrices for the preparation of drug-eluting stents. J Mater Chem. 2011; 21(22):8169–8177.13. Chamié D, Bezerra HG, Attizzani GF, Yamamoto H, Kanaya T, Stefano GT, et al. Incidence, predictors, morphological characteristics, and clinical outcomes of stent edge dissections detected by optical coherence tomography. JACC Cardiovasc Interv. 2013; 6(8):800–813. PMID: 23871510.14. Guagliumi G, Sirbu V, Musumeci G, Gerber R, Biondi-Zoccai G, Ikejima H, et al. Examination of the in vivo mechanisms of late drug-eluting stent thrombosis: findings from optical coherence tomography and intravascular ultrasound imaging. JACC Cardiovasc Interv. 2012; 5(1):12–20. PMID: 22230145.15. Moussa ID, Klein LW, Shah B, Mehran R, Mack MJ, Brilakis ES, et al. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). J Am Coll Cardiol. 2013; 62(17):1563–1570. PMID: 24135581.16. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007; 115(17):2344–2351. PMID: 17470709.17. Stone GW, Rizvi A, Newman W, Mastali K, Wang JC, Caputo R, et al. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N Engl J Med. 2010; 362(18):1663–1674. PMID: 20445180.18. Kim U, Lee CH, Jo JH, Lee HW, Choi YJ, Son JW, et al. A prospective, randomized comparison of promus everolimus-eluting and TAXUS Liberte paclitaxel-eluting stent systems in patients with coronary artery disease eligible for percutaneous coronary intervention: the PROMISE study. J Korean Med Sci. 2013; 28(11):1609–1614. PMID: 24265523.19. Garot P, Morice MC, Tresukosol D, Pocock SJ, Meredith IT, Abizaid A, et al. 2-Year outcomes of high bleeding risk patients after polymer-free drug-coated stents. J Am Coll Cardiol. 2017; 69(2):162–171. PMID: 27806919.20. Rozemeijer R, Stein M, Voskuil M, van den Bor R, Frambach P, Pereira B, et al. Randomized all-comers evaluation of a permanent polymer zotarolimus-eluting stent versus a polymer-free amphilimus-eluting stent. Circulation. 2019; 139(1):67–77. PMID: 30586704.21. Chiarito M, Sardella G, Colombo A, Briguori C, Testa L, Bedogni F, et al. Safety and efficacy of polymer-free drug-eluting stents. Circ Cardiovasc Interv. 2019; 12(2):e007311. PMID: 30767663.22. Windecker S, Latib A, Kedhi E, Kirtane AJ, Kandzari DE, Mehran R, et al. Polymer-based or polymer-free stents in patients at high bleeding risk. N Engl J Med. 2020; 382(13):1208–1218. PMID: 32050061.23. Kufner S, Ernst M, Cassese S, Joner M, Mayer K, Colleran R, et al. 10-Year outcomes from a randomized trial of polymer-free versus durable polymer drug-eluting coronary stents. J Am Coll Cardiol. 2020; 76(2):146–158. PMID: 32646563.24. Jensen LO, Maeng M, Raungaard B, Kahlert J, Ellert J, Jakobsen L, et al. Randomized comparison of the polymer-free biolimus-coated biofreedom stent with the ultrathin strut biodegradable polymer sirolimus-eluting orsiro stent in an all-comers population treated with percutaneous coronary intervention: the SORT OUT IX trial. Circulation. 2020; 141(25):2052–2063. PMID: 32434381.25. Byrne RA, Stone GW, Ormiston J, Kastrati A. Coronary balloon angioplasty, stents, and scaffolds. Lancet. 2017; 390(10096):781–792. PMID: 28831994.26. Song SJ, Park YJ, Park J, Cho MD, Kim JH, Jeong MH, et al. Preparation of a drug -eluting stent using a TiO2 film deposited by plasma enhanced chemical vapour deposition as a drug -combining matrix. J Mater Chem. 2010; 20(23):4792–4801.27. Hanawa T, Asami K, Asaoka K. Repassivation of titanium and surface oxide film regenerated in simulated bioliquid. J Biomed Mater Res. 1998; 40(4):530–538. PMID: 9599028.28. Park DS, Bae IH, Jeong MH, Lim KS, Sim DS, Hong YJ, et al. In vitro and in vivo evaluation of a novel polymer-free everolimus-eluting stent by nitrogen-doped titanium dioxide film deposition. Mater Sci Eng C. 2018; 91:615–623.29. Hyun DY, Han X, Park DS, Kim M, Park JK, Cho KH, et al. A novel polymer-free everolimus-eluting stent with a nitrogen-doped titanium dioxide film inhibits restenosis and thrombosis in a swine coronary model. Cardiol J. 2023; 30(6):881–891. PMID: 36790043.30. Yeung AC, Leon MB, Jain A, Tolleson TR, Spriggs DJ, Mc Laurin BT, et al. Clinical evaluation of the resolute zotarolimus-eluting coronary stent system in the treatment of de novo lesions in native coronary arteries: the RESOLUTE US clinical trial. J Am Coll Cardiol. 2011; 57(17):1778–1783. PMID: 21470813.31. Meredith IT, Worthley S, Whitbourn R, Walters DL, McClean D, Horrigan M, et al. Clinical and angiographic results with the next-generation resolute stent system: a prospective, multicenter, first-in-human trial. JACC Cardiovasc Interv. 2009; 2(10):977–985. PMID: 19850258.32. Stone GW, Midei M, Newman W, Sanz M, Hermiller JB, Williams J, et al. Comparison of an everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease: a randomized trial. JAMA. 2008; 299(16):1903–1913. PMID: 18430909.33. Serruys PW, Ong AT, Piek JJ, Neumann FJ, van der Giessen WJ, Wiemer M, et al. A randomized comparison of a durable polymer everolimus-eluting stent with a bare metal coronary stent: the SPIRIT first trial. EuroIntervention. 2005; 1(1):58–65. PMID: 19758878.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Preclinical Evaluation of a Novel Polymer-free Everolimus-eluting Stent in a Mid-term Porcine Coronary Restenosis Model
- Coronary Stent Thrombosis: Current Insights into New Drug-Eluting Stent Designs
- Kounis Syndrome Presenting as Very Late Stent Thrombosis in an Everolimus-Eluting Stent Following Wasp Stings
- Drug-Eluting Stent Strut Fracture as a Cause of Restenosis
- Very Late Stent Thrombosis Related to Fracture of a Sirolimus-Eluting Stent