J Korean Med Sci.
2024 Sep;39(35):e237. 10.3346/jkms.2024.39.e237.
Viral, Immunologic, and Laboratory Parameters in Patients With and Without Post-Acute Sequelae of SARS-CoV-2 Infection (PASC)
- Affiliations
-
- 1Department of Infectious Diseases, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Microbiology, Hanyang University College of Medicine, Seoul, Korea
- KMID: 2559193
- DOI: http://doi.org/10.3346/jkms.2024.39.e237
Abstract
- Background
The pathophysiological mechanisms underlying the post-acute sequelae of severe acute respiratory syndrome coronavirus 2 infection (PASC) are not well understood. Our study aimed to investigate various aspects of theses mechanisms, including viral persistence, immunological responses, and laboratory parameters in patients with and without PASC.
Methods
We prospectively enrolled adults aged ≥ 18 years diagnosed with coronavirus disease 2019 (COVID-19) between August 2022 and July 2023. Blood samples were collected at three time-points: within one month of diagnosis (acute phase) and at 1 month, and 3 months post-diagnosis. Following a recent well-designed definition of PASC, PASC patients were defined as those with a questionnaire-based PASC score ≥ 12 persisting for at least 4 weeks after the initial COVID-19 diagnosis.
Results
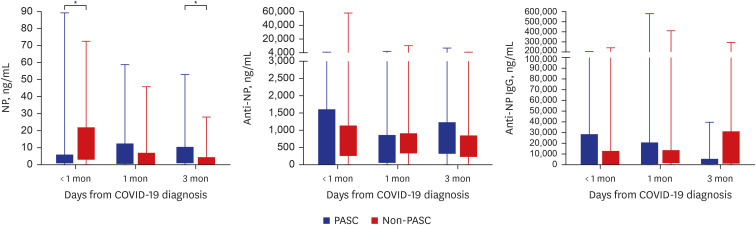
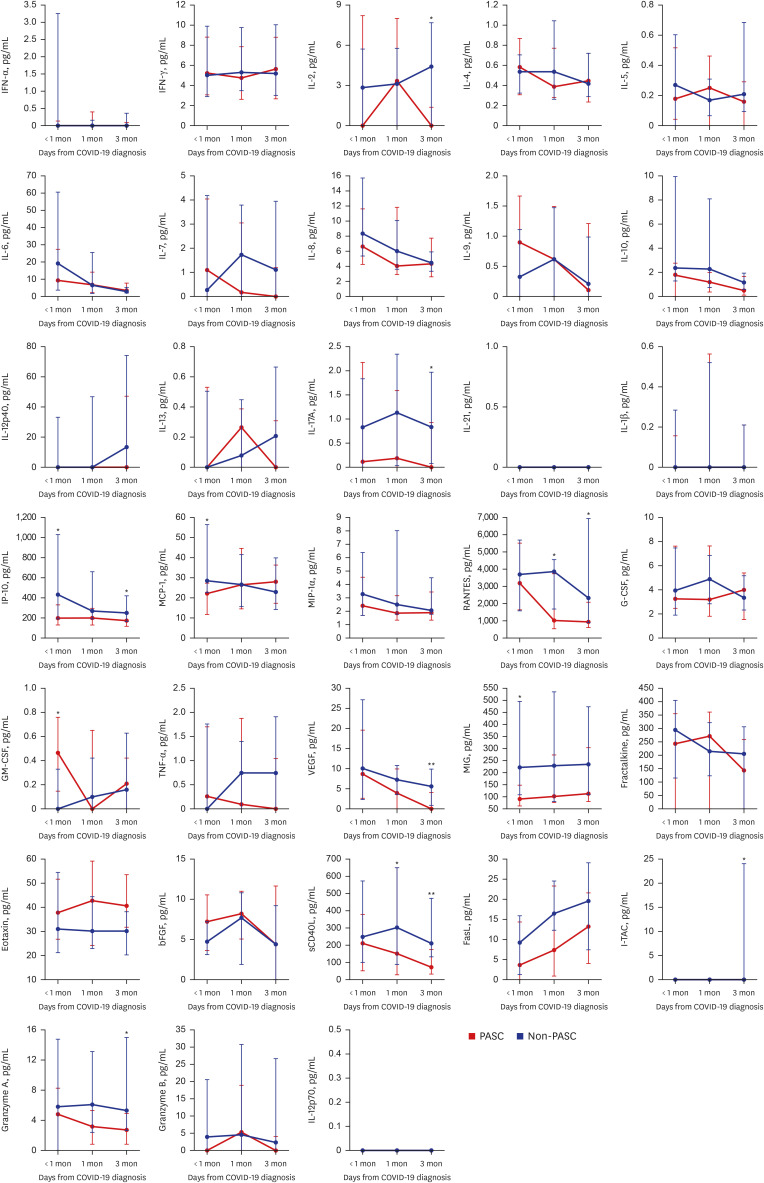
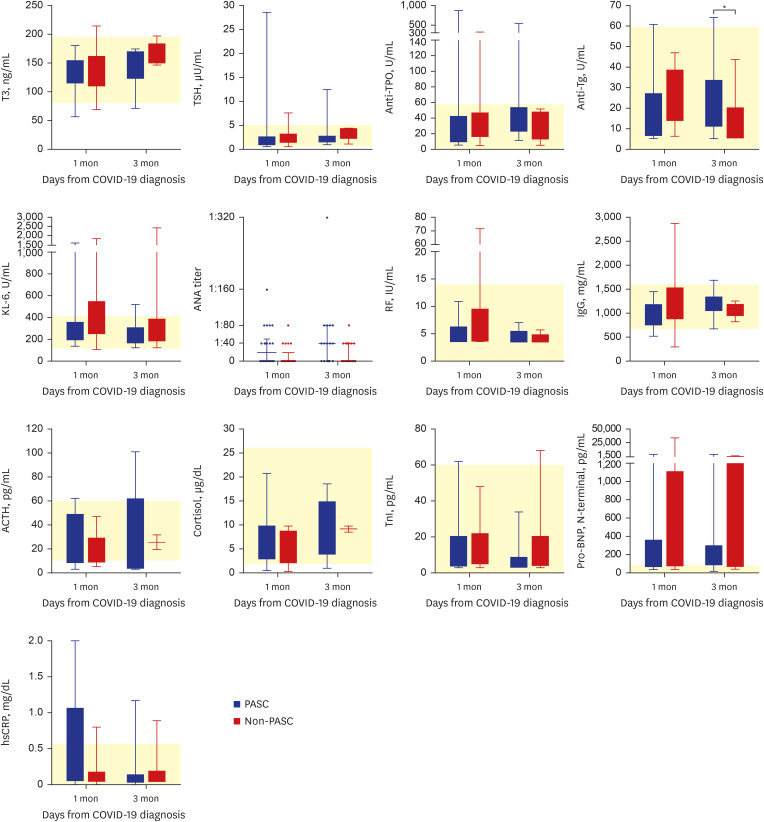
Of 57 eligible COVID-19 patients, 29 (51%) had PASC, and 28 (49%) did not. The PASC group had significantly higher nucleocapsid protein (NP) antigenemia 3 months after COVID-19 diagnosis (P = 0.022). Furthermore, several cytokines, including IL-2, IL-17A, VEGF, RANTES, sCD40L, IP-10, I-TAC, and granzyme A, were markedly elevated in the PASC group 1 and/or 3 month(s) after COVID-19 diagnosis. In contrast, the median values of several serological markers, including thyroid markers, autoimmune indicators, and stress-related hormones, were within the normal range.
Conclusion
Levels of NP antigen and of various cytokines involved in immune responses become significantly elevated over time after COVID-19 diagnosis in PASC patients compared to non-PASC patients. This suggests that PASC is associated with prolonged immune dysregulation resulting from heightened antigenic stimulation.
Keyword
Figure
Reference
-
1. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; 382(18):1708–1720. PMID: 32109013.2. Sneller MC, Liang CJ, Marques AR, Chung JY, Shanbhag SM, Fontana JR, et al. A longitudinal study of COVID-19 sequelae and immunity: baseline findings. Ann Intern Med. 2022; 175(7):969–979. PMID: 35605238.3. Swank Z, Senussi Y, Manickas-Hill Z, Yu XG, Li JZ, Alter G, et al. Persistent circulating severe acute respiratory syndrome coronavirus 2 spike is associated with post-acute coronavirus disease 2019 sequelae. Clin Infect Dis. 2023; 76(3):e487–e490. PMID: 36052466.4. Peluso MJ, Deeks SG, Mustapic M, Kapogiannis D, Henrich TJ, Lu S, et al. SARS-CoV-2 and mitochondrial proteins in neural-derived exosomes of COVID-19. Ann Neurol. 2022; 91(6):772–781. PMID: 35285072.5. Schultheiß C, Willscher E, Paschold L, Gottschick C, Klee B, Henkes SS, et al. The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep Med. 2022; 3(6):100663. PMID: 35732153.6. Torres-Ruiz J, Lomelín-Gascón J, Lira Luna J, Vargas-Castro AS, Pérez-Fragoso A, Nuñez-Aguirre M, et al. Novel clinical and immunological features associated with persistent post-acute sequelae of COVID-19 after six months of follow-up: a pilot study. Infect Dis (Lond). 2023; 55(4):243–254. PMID: 36637466.7. Thaweethai T, Jolley SE, Karlson EW, Levitan EB, Levy B, McComsey GA, et al. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA. 2023; 329(22):1934–1946. PMID: 37278994.8. Kwon JS, Kim JY, Kim MC, Park SY, Kim BN, Bae S, et al. Factors of severity in patients with COVID-19: cytokine/chemokine concentrations, viral load, and antibody responses. Am J Trop Med Hyg. 2020; 103(6):2412–2418. PMID: 33124544.9. Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005; 47(4):458–472. PMID: 16161804.10. Verkerke HP, Damhorst GL, Graciaa DS, McLendon K, O’Sick W, Robichaux C, et al. Nucleocapsid antigenemia is a marker of acute SARS-CoV-2 infection. J Infect Dis. 2022; 226(9):1577–1587. PMID: 35877413.11. Choi YJ, Seo YB, Seo JW, Lee J, Nham E, Seong H, et al. Effectiveness of antiviral therapy on long COVID: a systematic review and meta-analysis. J Clin Med. 2023; 12(23):7375. PMID: 38068427.12. Wolff G, Melia CE, Snijder EJ, Bárcena M. Double-membrane vesicles as platforms for viral replication. Trends Microbiol. 2020; 28(12):1022–1033. PMID: 32536523.13. V’kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021; 19(3):155–170. PMID: 33116300.14. Kreye J, Reincke SM, Prüss H. Do cross-reactive antibodies cause neuropathology in COVID-19? Nat Rev Immunol. 2020; 20(11):645–646. PMID: 33024283.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Letter to the Editor: Before Characterising Post-Acute Sequelae of SARS-CoV-2 Infection by Viral and Immunologic Parameters, Alternative Causes of Their Increase Must Be Ruled Out
- Post-acute sequelae of SARS-CoV-2 syndrome presenting as postural orthostatic tachycardia syndrome
- Erratum: Correction of Figure in the Article “Viral, Immunologic, and Laboratory Parameters in Patients With and Without Post-Acute Sequelae of SARS-CoV-2 Infection (PASC)”
- Understandings and Prospects of Laboratory Diagnosis of SARS-CoV-2
- SARS-CoV-2 in the Prostate: Immunohistochemical and Ultrastructural Studies