Korean Circ J.
2024 Sep;54(9):565-576. 10.4070/kcj.2024.0038.
Korean Multicenter Registry of ELUVIA Stent for Femoropopliteal Artery Disease: K-ELUVIA Registry
- Affiliations
-
- 1Division of Cardiology, Severance Cardiovascular Hospital, Yonsei University College of Medicine, Seoul, Korea
- 2Division of Cardiology, Cardiovascular Center, Korea University Guro Hospital, Seoul, Korea
- 3Division of Cardiology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 4Division of Cardiology, National Health Insurance Service Ilsan Hospital, Goyang, Korea
- 5Division of Cardiology, Wonju Severance Hospital, Yonsei University Wonju College of Medicine, Wonju, Korea
- 6Division of Cardiology, Department of Internal Medicine, Dankook University College of Medicine, Cheonan, Korea
- 7Division of Cardiology, Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- KMID: 2559129
- DOI: http://doi.org/10.4070/kcj.2024.0038
Abstract
- Background and Objectives
The K-ELUVIA study aimed to investigate the clinical effectiveness and safety of Eluvia™, a polymer-coated, paclitaxel-eluting stent, for femoropopliteal artery disease using data from a prospective Korean multicenter registry.
Methods
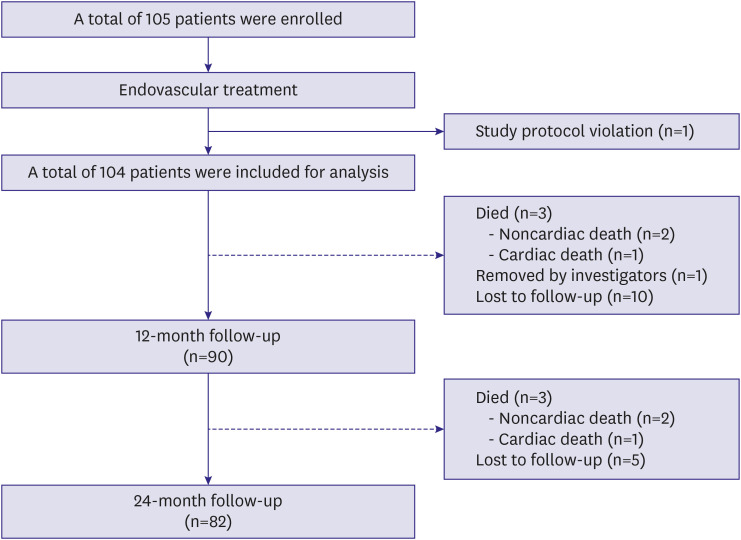
A total of 105 patients with femoropopliteal artery disease who received endovascular treatment (EVT) with Eluvia™ stents at 7 Korean sites were enrolled in a prospective cohort and followed for 2 years. The primary endpoint was the 2-year clinical patency. The secondary endpoint was 2-year freedom from clinically driven target lesion revascularization (TLR).
Results
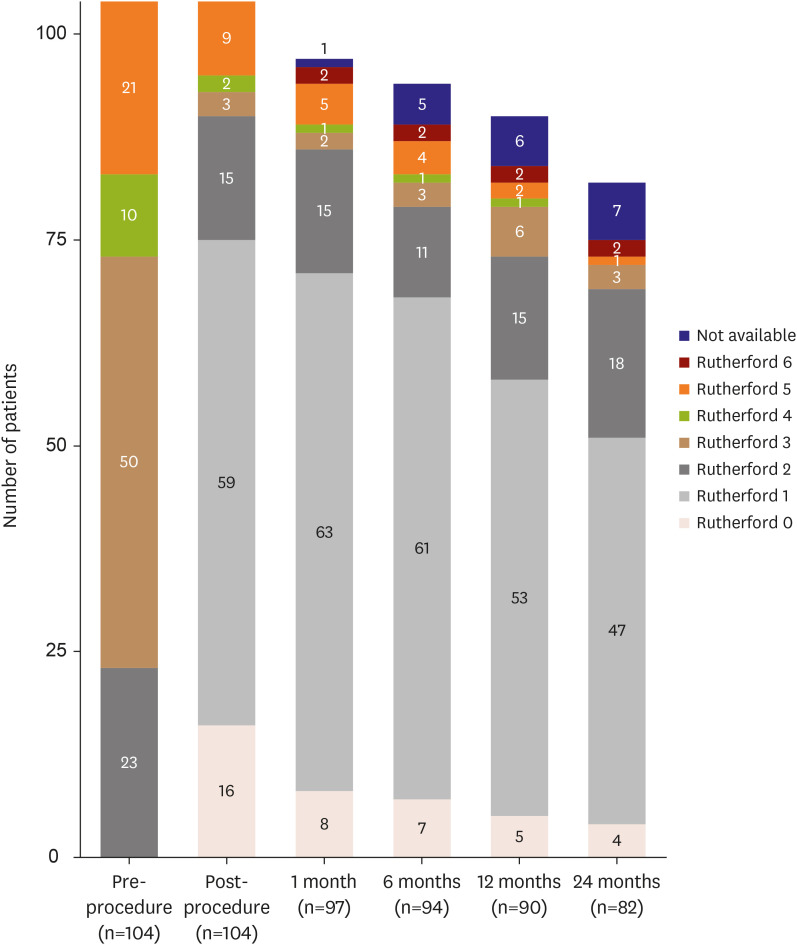
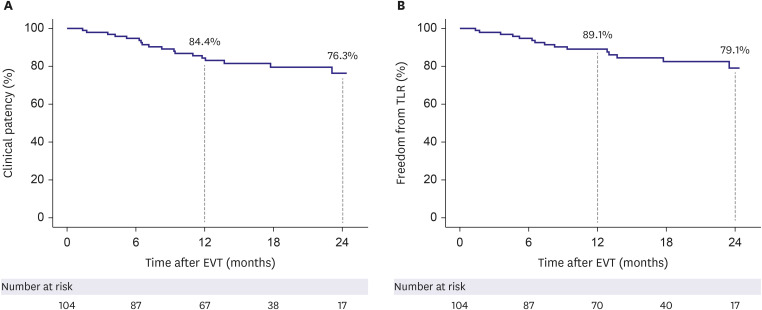
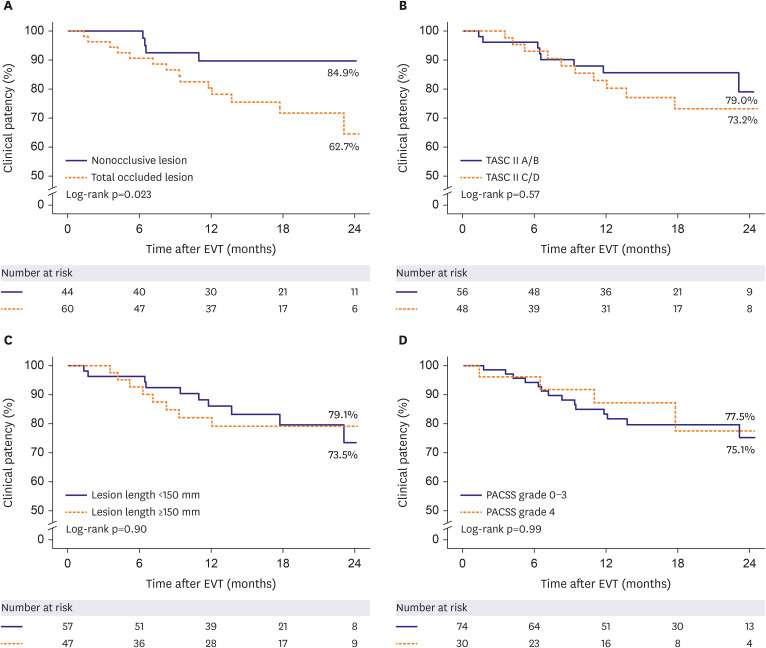
Mean patient age was 68.2±10.4 years, and most patients (82.7%) were male. Mean lesion length was 168.3±117.6 mm. Chronic total occlusion was found in 57.7% of patients. Trans-Atlantic Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) type C or D lesions were present in 46.1% of patients. Procedural success was achieved in 99.0% of patients. The clinical patency rate was 84.4% at 1 year after EVT and 76.3% at 2 years post-EVT. The freedom from TLR rate was 89.1% at 1 year after EVT and 79.1% at 2 years post-EVT. Chronic total occlusion (hazard ratio [HR], 3.53; 95% confidence interval [CI], 1.08–11.67; p=0.039) and smaller mean stent diameter (HR, 0.40; 95% CI, 0.16– 0.98; p=0.044) were identified as independent predictors of loss of clinical patency at 2 years.
Conclusions
The K-ELUVIA study demonstrated favorable 2-year clinical effectiveness and safety outcomes of Eluvia stent for femoropopliteal artery lesions in real-world practice.
Keyword
Figure
Reference
-
1. Ko YG, Ahn CM, Min PK, et al. Baseline characteristics of a retrospective patient cohort in the Korean Vascular Intervention Society Endovascular Therapy in Lower Limb Artery Diseases (K-VIS ELLA) Registry. Korean Circ J. 2017; 47:469–476. PMID: 28765738.2. Poulson W, Kamenskiy A, Seas A, Deegan P, Lomneth C, MacTaggart J. Limb flexion-induced axial compression and bending in human femoropopliteal artery segments. J Vasc Surg. 2018; 67:607–613. PMID: 28526560.3. Acin F, de Haro J, Bleda S, Varela C, Esparza L. Primary nitinol stenting in femoropopliteal occlusive disease: a meta-analysis of randomized controlled trials. J Endovasc Ther. 2012; 19:585–595. PMID: 23046322.4. Tepe G, Zeller T, Albrecht T, et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med. 2008; 358:689–699. PMID: 18272892.5. Dake MD, Ansel GM, Jaff MR, et al. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results. Circ Cardiovasc Interv. 2011; 4:495–504. PMID: 21953370.6. Dake MD, Ansel GM, Jaff MR, et al. Sustained safety and effectiveness of paclitaxel-eluting stents for femoropopliteal lesions: 2-year follow-up from the Zilver PTX randomized and single-arm clinical studies. J Am Coll Cardiol. 2013; 61:2417–2427. PMID: 23583245.7. Dake MD, Ansel GM, Bosiers M, et al. Paclitaxel-coated Zilver PTX drug-eluting stent treatment does not result in increased long-term all-cause mortality compared to uncoated devices. Cardiovasc Intervent Radiol. 2020; 43:8–19. PMID: 31502026.8. Kichikawa K, Ichihashi S, Yokoi H, et al. Zilver PTX post-market surveillance study of paclitaxel-eluting stents for treating femoropopliteal artery disease in Japan: 2-year results. Cardiovasc Intervent Radiol. 2019; 42:358–364. PMID: 30411151.9. Iida O, Takahara M, Soga Y, et al. The characteristics of in-stent restenosis after drug-eluting stent implantation in femoropopliteal lesions and 1-year prognosis after repeat endovascular therapy for these lesions. JACC Cardiovasc Interv. 2016; 9:828–834. PMID: 27101908.10. Iida O, Takahara M, Soga Y, et al. 1-Year results of the ZEPHYR registry (Zilver PTX for the femoral artery and proximal popliteal artery): predictors of restenosis. JACC Cardiovasc Interv. 2015; 8:1105–1112. PMID: 26117463.11. Gray WA, Keirse K, Soga Y, et al. A polymer-coated, paclitaxel-eluting stent (Eluvia) versus a polymer-free, paclitaxel-coated stent (Zilver PTX) for endovascular femoropopliteal intervention (IMPERIAL): a randomised, non-inferiority trial. Lancet. 2018; 392:1541–1551. PMID: 30262332.12. Rocha-Singh KJ, Zeller T, Jaff MR. Peripheral arterial calcification: prevalence, mechanism, detection, and clinical implications. Catheter Cardiovasc Interv. 2014; 83:E212–E220. PMID: 24402839.13. Gouëffic Y, Torsello G, Zeller T, et al. Efficacy of a drug-eluting stent versus bare metal stents for symptomatic femoropopliteal peripheral artery disease: primary results of the EMINENT randomized trial. Circulation. 2022; 146:1564–1576. PMID: 36254728.14. Müller-Hülsbeck S, Benko A, Soga Y, et al. Two-year efficacy and safety results from the IMPERIAL randomized study of the Eluvia polymer-coated drug-eluting stent and the Zilver PTX polymer-free drug-coated stent. Cardiovasc Intervent Radiol. 2021; 44:368–375. PMID: 33225377.15. Iida O, Takahara M, Soga Y, et al. 1-Year outcomes of fluoropolymer-based drug-eluting stent in femoropopliteal practice: predictors of restenosis and aneurysmal degeneration. JACC Cardiovasc Interv. 2022; 15:630–638. PMID: 35331454.16. Stavroulakis K, Torsello G, Bosiers M, Argyriou A, Tsilimparis N, Bisdas T. 2-Year outcomes of the Eluvia drug-eluting stent for the treatment of complex femoropopliteal lesions. JACC Cardiovasc Interv. 2021; 14:692–701. PMID: 33736776.17. Ichihashi S, Takahara M, Yamaoka T, et al. Drug eluting versus covered stent for femoropopliteal artery lesions: results of the ULTIMATE study. Eur J Vasc Endovasc Surg. 2022; 64:359–366. PMID: 35671936.18. Dake MD, Fanelli F, Lottes AE, et al. Prediction model for freedom from TLR from a multi-study analysis of long-term results with the Zilver PTX drug-eluting peripheral stent. Cardiovasc Intervent Radiol. 2021; 44:196–206. PMID: 33025243.19. Tsujimura T, Iida O, Asai M, et al. Aneurysmal degeneration of fluoropolymer-coated paclitaxel-eluting stent in the superficial femoral artery: a rising concern. CVIR Endovasc. 2021; 4:56. PMID: 34216312.20. Bisdas T, Beropoulis E, Argyriou A, Torsello G, Stavroulakis K. 1-Year all-comers analysis of the Eluvia drug-eluting stent for long femoropopliteal lesions after suboptimal angioplasty. JACC Cardiovasc Interv. 2018; 11:957–966. PMID: 29798772.21. Soga Y, Ando K.. “Low Echoic Area” around stent after bare and drug-coated stenting or stent graft placement for superficial femoral artery disease. SAGE Open Med Case Rep. 2021; 9:2050313X211014519.22. Holden A, Gouëffic Y, Gray WA, Davis EJ, Weinberg I, Jaff MR. Hypoechoic halo imaging findings following femoropopliteal artery stent implantation: risk factors and clinical outcomes. JACC Cardiovasc Interv. 2023; 16:1654–1664. PMID: 37438033.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential efficacy between stenting and plain balloon angioplasty for femoropopliteal disease with or without total occlusion
- Treatment of Femoropopliteal Artery In-stent Restenosis
- Development of Registry System for the Construction of Coronary Intervention Database
- Femoropopliteal Artery Stent Fracture with Recurrent In-Stent Reocclusion and Aneurysm Formation: Successful Treatment with Self-Expandable Viabahn Endoprosthesis
- Current Status of the Korean Venous Thromboembolism Registry