Diabetes Metab J.
2024 Sep;48(5):983-992. 10.4093/dmj.2023.0149.
Glycemic Control and Retinal Microvascular Changes in Type 2 Diabetes Mellitus Patients without Clinical Retinopathy
- Affiliations
-
- 1Department of Ophthalmology, Dongguk University Ilsan Hospital, Goyang, Korea
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, Dongguk University Ilsan Hospital, Goyang, Korea
- 3Department of Ophthalmology, Seoul National University Hospital, Seoul, Korea
- KMID: 2559007
- DOI: http://doi.org/10.4093/dmj.2023.0149
Abstract
- Background
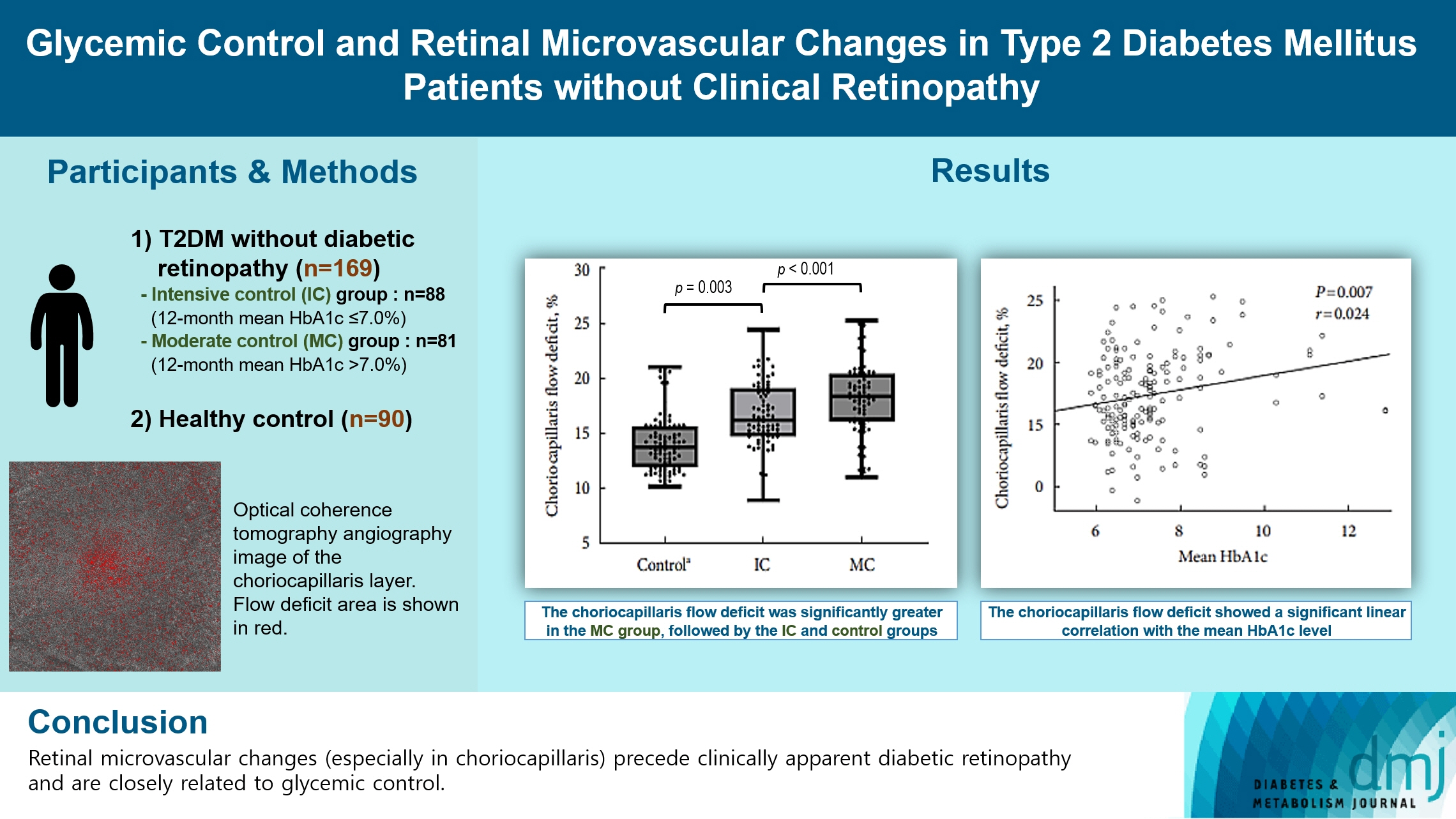
To investigate the association of glycemic control and retinal microvascular changes in patients with type 2 diabetes mellitus (T2DM) without diabetic retinopathy (DR).
Methods
This retrospective, observational, cohort study included patients with T2DM without DR. The patients were categorized into intensive control (IC; mean glycosylated hemoglobin [HbA1c] ≤7.0%) and moderate control (MC; mean HbA1c >7.0%) groups. Optical coherence tomography (OCT) and swept-source OCT angiography (OCTA) image parameters were compared between three groups, including healthy controls.
Results
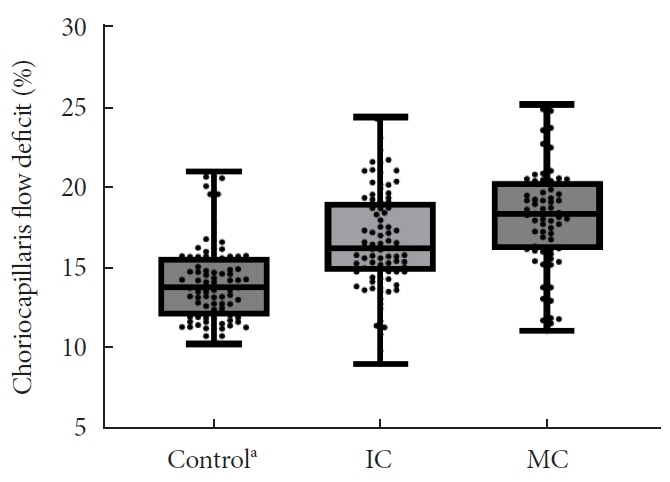
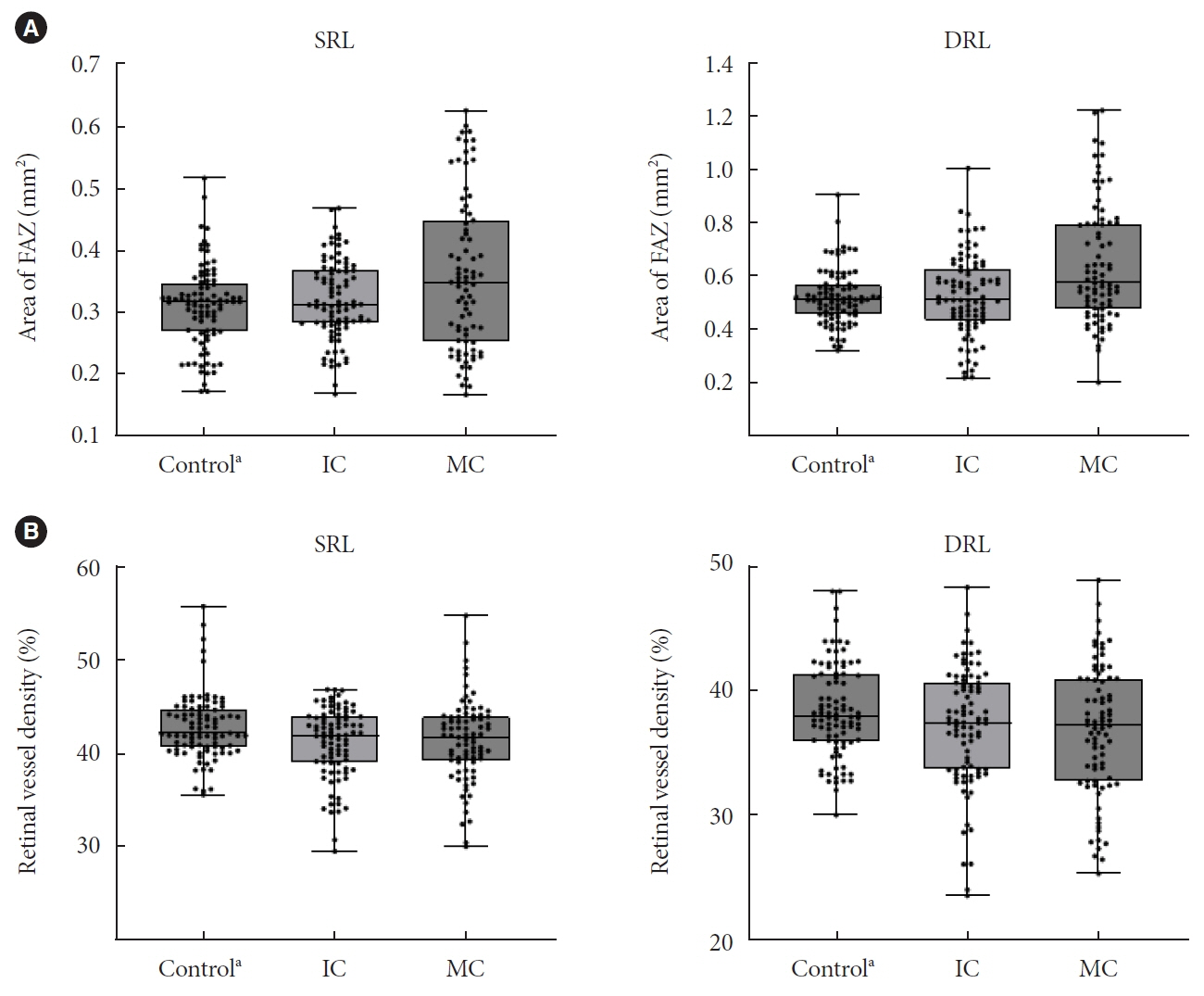
In total, 259 eyes of 259 participants (88 IC, 81 MC, and 90 controls) were included. The foveal avascular zone area was significantly larger in the MC group than IC and control groups (all P<0.05). The IC group had lower vessel density in the superficial retinal layer and deep retinal layer than the controls (all P<0.05). The choriocapillaris (CC) flow deficit (FD) was significantly greater in the MC group than in the IC and control groups (18.2%, 16.7%, and 14.2%, respectively; all P<0.01). In multivariate regression analysis, CC-FD was associated with the mean HbA1c level (P=0.008). There were no significant differences in OCT parameters among the groups.
Conclusion
OCTA revealed that early CC impairment is associated with HbA1c levels; the CC changes precede clinically apparent DR. The OCTA parameters differed among the groups according to the degree of glycemic control. Our results suggest that microvascular changes precede DR and are closely related to glycemic control.
Keyword
Figure
Reference
-
1. Kim JH, Kim DJ, Jang HC, Choi SH. Epidemiology of microand macrovascular complications of type 2 diabetes in Korea. Diabetes Metab J. 2011; 35:571–7.
Article2. Lee SH, Park SY, Choi CS. Insulin resistance: from mechanisms to therapeutic strategies. Diabetes Metab J. 2022; 46:15–37.
Article3. Rosen RB, Andrade Romo JS, Krawitz BD, Mo S, Fawzi AA, Linderman RE, et al. Earliest evidence of preclinical diabetic retinopathy revealed using optical coherence tomography angiography perfused capillary density. Am J Ophthalmol. 2019; 203:103–15.
Article4. Ishibashi F, Kosaka A, Tavakoli M. The impact of glycemic control on retinal photoreceptor layers and retinal pigment epithelium in patients with type 2 diabetes without diabetic retinopathy: a follow-up study. Front Endocrinol (Lausanne). 2021; 12:614161.
Article5. Lee MW, Lee WH, Ryu CK, Kim TY, Lim HB, Lee YH, et al. Effects of prolonged type 2 diabetes on the inner retinal layer and macular microvasculature: an optical coherence tomography angiography study. J Clin Med. 2020; 9:1849.
Article6. Vujosevic S, Muraca A, Gatti V, Masoero L, Brambilla M, Cannillo B, et al. Peripapillary microvascular and neural changes in diabetes mellitus: an OCT-angiography study. Invest Ophthalmol Vis Sci. 2018; 59:5074–81.
Article7. Massin P, Marre M. Fundus photography for the screening for diabetic retinopathy. Diabetes Metab. 2002; 28:151–5.8. Koleva-Georgieva D, Sivkova N. Assessment of serous macular detachment in eyes with diabetic macular edema by use of spectral-domain optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2009; 247:1461–9.
Article9. Lang GE. Optical coherence tomography findings in diabetic retinopathy. Dev Ophthalmol. 2007; 39:31–47.
Article10. Agemy SA, Scripsema NK, Shah CM, Chui T, Garcia PM, Lee JG, et al. Retinal vascular perfusion density mapping using optical coherence tomography angiography in normals and diabetic retinopathy patients. Retina. 2015; 35:2353–63.
Article11. Al-Sheikh M, Akil H, Pfau M, Sadda SR. Swept-source OCT angiography imaging of the foveal avascular zone and macular capillary network density in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2016; 57:3907–13.
Article12. Nesper PL, Soetikno BT, Zhang HF, Fawzi AA. OCT angiography and visible-light OCT in diabetic retinopathy. Vision Res. 2017; 139:191–203.
Article13. Yun JS, Lim TS, Cha SA, Ahn YB, Song KH, Choi JA, et al. Clinical course and risk factors of diabetic retinopathy in patients with type 2 diabetes mellitus in Korea. Diabetes Metab J. 2016; 40:482–93.
Article14. Azad N, Agrawal L, Bahn G, Emanuele NV, Reaven PD, Hayward R, et al. Eye outcomes in Veteran Affairs Diabetes Trial (VADT) after 17 years. Diabetes Care. 2021; 44:2397–402.
Article15. Ipp E, Kumar M. A clinical conundrum: intensifying glycemic control in the presence of advanced diabetic retinopathy. Diabetes Care. 2021; 44:2192–3.
Article16. Zahid S, Dolz-Marco R, Freund KB, Balaratnasingam C, Dansingani K, Gilani F, et al. Fractal dimensional analysis of optical coherence tomography angiography in eyes with diabetic retinopathy. Invest Ophthalmol Vis Sci. 2016; 57:4940–7.
Article17. Cicinelli MV, Rabiolo A, Marchese A, de Vitis L, Carnevali A, Querques L, et al. Choroid morphometric analysis in non-neovascular age-related macular degeneration by means of optical coherence tomography angiography. Br J Ophthalmol. 2017; 101:1193–200.
Article18. Chu Z, Cheng Y, Zhang Q, Zhou H, Dai Y, Shi Y, et al. Quantification of choriocapillaris with phansalkar local thresholding: pitfalls to avoid. Am J Ophthalmol. 2020; 213:161–76.
Article19. Zhang Q, Zheng F, Motulsky EH, Gregori G, Chu Z, Chen CL, et al. A novel strategy for quantifying choriocapillaris flow voids using swept-source OCT angiography. Invest Ophthalmol Vis Sci. 2018; 59:203–11.
Article20. Twomey PJ, Rayman G, Pledger DR. Implications of different DCCT-aligned HbA1c methods on GMS clinical indicators. Diabet Med. 2008; 25:97–100.
Article21. King P, Peacock I, Donnelly R. The UK prospective diabetes study (UKPDS): clinical and therapeutic implications for type 2 diabetes. Br J Clin Pharmacol. 1999; 48:643–8.
Article22. Wu TE, Su YW, Chen HS. Mean HbA1c and HbA1c variability are associated with differing diabetes-related complications in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2022; 192:110069.
Article23. Lee MW, Koo HM, Lee WH, Park JH, Lee YH, Kim JY. Impacts of systemic hypertension on the macular microvasculature in diabetic patients without clinical diabetic retinopathy. Invest Ophthalmol Vis Sci. 2021; 62:21.
Article24. Zhang B, Chou Y, Zhao X, Yang J, Chen Y. Early detection of microvascular impairments with optical coherence tomography angiography in diabetic patients without clinical retinopathy: a meta-analysis. Am J Ophthalmol. 2021; 222:226–37.
Article25. Singh C. Metabolism and vascular retinopathies: current perspectives and future directions. Diagnostics (Basel). 2022; 12:903.
Article26. Moreira-Neto CA, Moult EM, Fujimoto JG, Waheed NK, Ferrara D. Choriocapillaris loss in advanced age-related macular degeneration. J Ophthalmol. 2018; 2018:8125267.
Article27. Dimitrova G, Kato S, Tamaki Y, Yamashita H, Nagahara M, Sakurai M, et al. Choroidal circulation in diabetic patients. Eye (Lond). 2001; 15(Pt 5):602–7.
Article28. Wang W, Guo X, Chen Y, Xiong K, Gong X, Yuan M, et al. Choriocapillaris perfusion assessed using swept source optical coherence tomographic angiography and the severity of diabetic retinopathy. Br J Ophthalmol. 2023; 107:836–41.
Article29. Muir ER, Renteria RC, Duong TQ. Reduced ocular blood flow as an early indicator of diabetic retinopathy in a mouse model of diabetes. Invest Ophthalmol Vis Sci. 2012; 53:6488–94.
Article30. McLeod DS, Lutty GA. High-resolution histologic analysis of the human choroidal vasculature. Invest Ophthalmol Vis Sci. 1994; 35:3799–811.31. Zoungas S, Arima H, Gerstein HC, Holman RR, Woodward M, Reaven P, et al. Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol. 2017; 5:431–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Frequencies and Risk Factors for Microvascular Complications in Patients with Type 1 Diabetes Mellitus
- Glycemic targets in patients with diabetes
- Clinical Analysis of Diabetic Retinopathy According to the Type of Diabetes Mellitus
- Relationship between Glycemic Control and Diabetic Retinopathy
- Diagnosis and Glycemic Control of Type 1 Diabetes