Diabetes Metab J.
2024 Sep;48(5):960-970. 10.4093/dmj.2023.0039.
Biologically Informed Polygenic Scores for Brain Insulin Receptor Network Are Associated with Cardiometabolic Risk Markers and Diabetes in Women
- Affiliations
-
- 1Folkhälsan Research Center, Helsinki, Finland
- 2Department of General Practice and Primary Health Care, Helsinki University Hospital, University of Helsinki, Helsinki, Finland
- 3Department of Psychiatry, Faculty of Medicine, McGill University, Verdun, QC, Canada
- 4Ludmer Center for Neuroinformatic and Mental Health, Douglas Mental Health University Institute, McGill University, Verdun, QC, Canada
- 5Gerontology Research Center and Faculty of Sport and Health Sciences, University of Jyväskylä, Jyväskylä, Finland
- 6Department of Psychology and Logopedics, University of Helsinki, Helsinki, Finland
- 7Turku Institute for Advanced Studies, University of Turku, Turku, Finland
- 8Department of Clinical Chemistry and Haematology, Helsinki University Hospital, Faculty of Medicine, University of Helsinki, Helsinki, Finland
- 9Department of Clinical Chemistry and Obstetrics and Gynecology, Helsinki University Hospital, University of Helsinki, Helsinki, Finland
- 10Department of Obstetrics & Gynecology and Human Potential Translational Research Program, Yong Loo Lin School of Medicine, National University of Singapore, Singapore
- 11Singapore Institute for Clinical Sciences (SICS), Agency for Science, Technology and Research (A*STAR), Singapore
- KMID: 2559005
- DOI: http://doi.org/10.4093/dmj.2023.0039
Abstract
- Background
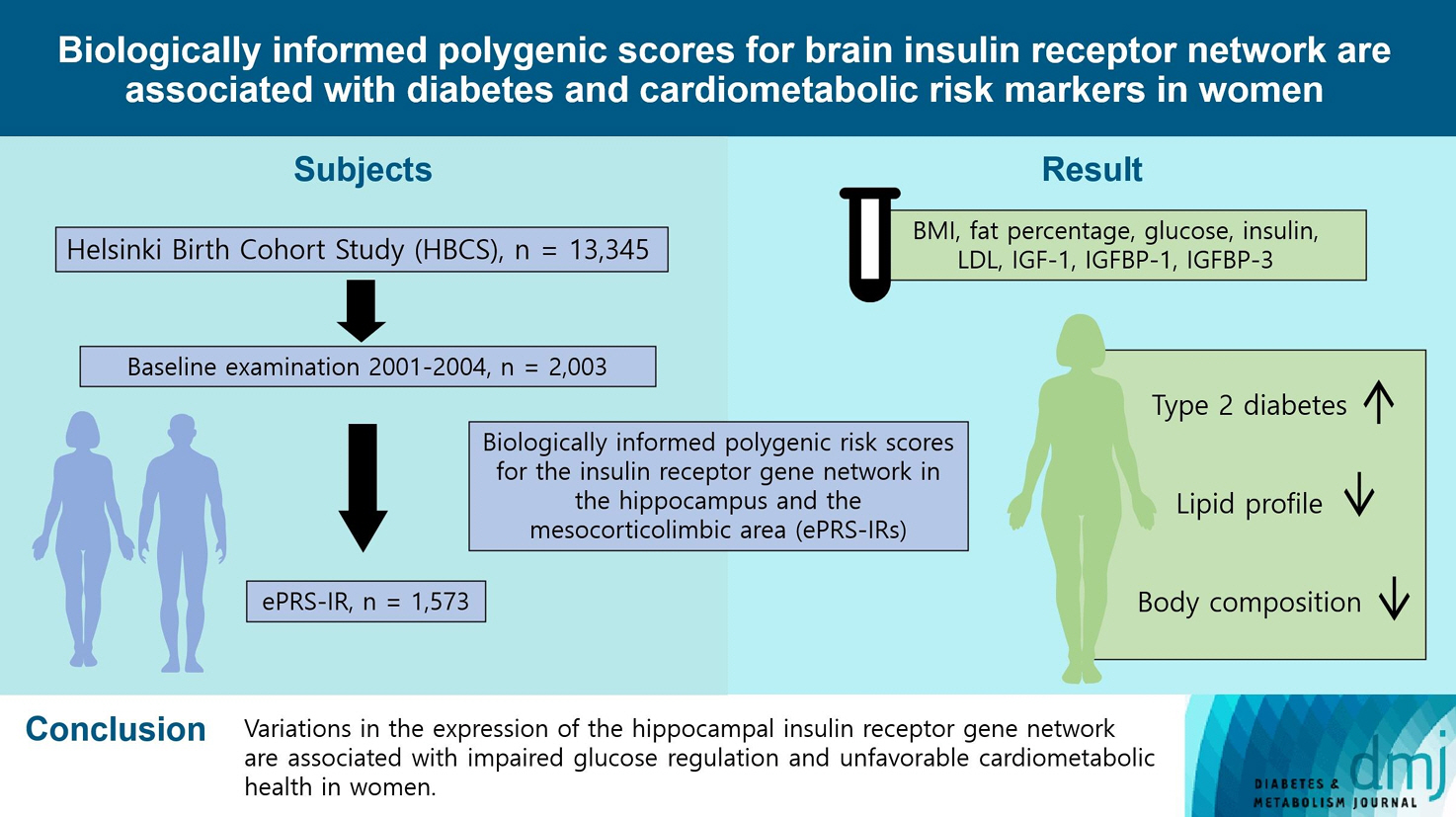
To investigate associations between variations in the co-expression-based brain insulin receptor polygenic score and cardiometabolic risk factors and diabetes mellitus.
Methods
This cross-sectional study included 1,573 participants from the Helsinki Birth Cohort Study. Biologically informed expression-based polygenic risk scores for the insulin receptor gene network were calculated for the hippocampal (hePRS-IR) and the mesocorticolimbic (mePRS-IR) regions. Cardiometabolic markers included body composition, waist circumference, circulating lipids, insulin-like growth factor 1 (IGF-1), and insulin-like growth factor-binding protein 1 and 3 (IGFBP-1 and -3). Glucose and insulin levels were measured during a standardized 2-hour 75 g oral glucose tolerance test and impaired glucose regulation status was defined by the World Health Organization 2019 criteria. Analyzes were adjusted for population stratification, age, smoking, alcohol consumption, socioeconomic status, chronic diseases, birth weight, and leisure-time physical activity.
Results
Multinomial logistic regression indicated that one standard deviation increase in hePRS-IR was associated with increased risk of diabetes mellitus in all participants (adjusted relative risk ratio, 1.17; 95% confidence interval, 1.01 to 1.35). In women, higher hePRS-IR was associated with greater waist circumference and higher body fat percentage, levels of glucose, insulin, total cholesterol, low-density lipoprotein cholesterol, triglycerides, apolipoprotein B, insulin, and IGFBP-1 (all P≤0.02). The mePRS-IR was associated with decreased IGF-1 level in women (P=0.02). No associations were detected in men and studied outcomes.
Conclusion
hePRS-IR is associated with sex-specific differences in cardiometabolic risk factor profiles including impaired glucose regulation, abnormal metabolic markers, and unfavorable body composition in women.
Figure
Reference
-
1. Pomytkin I, Costa-Nunes JP, Kasatkin V, Veniaminova E, Demchenko A, Lyundup A, et al. Insulin receptor in the brain: mechanisms of activation and the role in the CNS pathology and treatment. CNS Neurosci Ther. 2018; 24:763–74.
Article2. Grillo CA, Woodruff JL, Macht VA, Reagan LP. Insulin resistance and hippocampal dysfunction: disentangling peripheral and brain causes from consequences. Exp Neurol. 2019; 318:71–7.
Article3. Landau BR, Takaoka Y, Abrams MA, Genuth SM, van Houten M, Posner BI, et al. Binding of insulin by monkey and pig hypothalamus. Diabetes. 1983; 32:284–91.
Article4. Hill JM, Lesniak MA, Pert CB, Roth J. Autoradiographic localization of insulin receptors in rat brain: prominence in olfactory and limbic areas. Neuroscience. 1986; 17:1127–38.
Article5. Woods CA, Guttman ZR, Huang D, Kolaric RA, Rabinowitsch AI, Jones KT, et al. Insulin receptor activation in the nucleus accumbens reflects nutritive value of a recently ingested meal. Physiol Behav. 2016; 159:52–63.
Article6. Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018; 98:2133–223.
Article7. Kullmann S, Valenta V, Wagner R, Tschritter O, Machann J, Haring HU, et al. Brain insulin sensitivity is linked to adiposity and body fat distribution. Nat Commun. 2020; 11:1841.
Article8. Chen W, Balland E, Cowley MA. Hypothalamic insulin resistance in obesity: effects on glucose homeostasis. Neuroendocrinology. 2017; 104:364–81.
Article9. Banks WA, Jaspan JB, Kastin AJ. Selective, physiological transport of insulin across the blood-brain barrier: novel demonstration by species-specific radioimmunoassays. Peptides. 1997; 18:1257–62.
Article10. Kaiyala KJ, Prigeon RL, Kahn SE, Woods SC, Schwartz MW. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes. 2000; 49:1525–33.
Article11. Bahri S, Horowitz M, Malbert CH. Inward glucose transfer accounts for insulin-dependent increase in brain glucose metabolism associated with diet-induced obesity. Obesity (Silver Spring). 2018; 26:1322–31.
Article12. Rebelos E, Bucci M, Karjalainen T, Oikonen V, Bertoldo A, Hannukainen JC, et al. Insulin resistance is associated with enhanced brain glucose uptake during euglycemic hyperinsulinemia: a large-scale PET cohort. Diabetes Care. 2021; 44:788–94.
Article13. Kleinridders A, Ferris HA, Cai W, Kahn CR. Insulin action in brain regulates systemic metabolism and brain function. Diabetes. 2014; 63:2232–43.
Article14. Wardelmann K, Blumel S, Rath M, Alfine E, Chudoba C, Schell M, et al. Insulin action in the brain regulates mitochondrial stress responses and reduces diet-induced weight gain. Mol Metab. 2019; 21:68–81.
Article15. Novak V, Milberg W, Hao Y, Munshi M, Novak P, Galica A, et al. Enhancement of vasoreactivity and cognition by intranasal insulin in type 2 diabetes. Diabetes Care. 2014; 37:751–9.
Article16. Hari Dass SA, McCracken K, Pokhvisneva I, Chen LM, Garg E, Nguyen TT, et al. A biologically-informed polygenic score identifies endophenotypes and clinical conditions associated with the insulin receptor function on specific brain regions. EBioMedicine. 2019; 42:188–202.
Article17. GTEx Consortium. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013; 45:580–5.18. Silveira PP, Meaney MJ. Examining the biological mechanisms of human mental disorders resulting from gene-environment interdependence using novel functional genomic approaches. Neurobiol Dis. 2023; 178:106008.
Article19. von Bondorff MB, Tormakangas T, Salonen M, von Bonsdorff ME, Osmond C, Kajantie E, et al. Early life origins of all-cause and cause-specific disability pension: findings from the Helsinki Birth Cohort Study. PLoS One. 2015; 10:e0122134.
Article20. Koistinen H, Koistinen R, Selenius L, Ylikorkala Q, Seppala M. Effect of marathon run on serum IGF-I and IGF-binding protein 1 and 3 levels. J Appl Physiol (1985). 1996; 80:760–4.
Article21. World Health Organization. Classification of diabetes mellitus. Geneva: WHO;2019.22. Bedogni G, Malavolti M, Severi S, Poli M, Mussi C, Fantuzzi AL, et al. Accuracy of an eight-point tactile-electrode impedance method in the assessment of total body water. Eur J Clin Nutr. 2002; 56:1143–8.
Article23. Koistinen H, Seppala M, Koistinen R. Different forms of insulin-like growth factor-binding protein-3 detected in serum and seminal plasma by immunofluorometric assay with monoclonal antibodies. Clin Chem. 1994; 40:531–6.
Article24. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28:412–9.
Article25. Song Y, Manson JE, Tinker L, Howard BV, Kuller LH, Nathan L, et al. Insulin sensitivity and insulin secretion determined by homeostasis model assessment and risk of diabetes in a multiethnic cohort of women: the Women’s Health Initiative Observational Study. Diabetes Care. 2007; 30:1747–52.
Article26. Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med. 1994; 11:286–92.
Article27. Seltzer HS, Allen EW, Herron AL Jr, Brennan MT. Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest. 1967; 46:323–35.
Article28. Central Statistical Office of Finland. Classification of socio-economic group: handbooks 17. 17th ed. Helsinki: Central Statistical Office of Finland;1989.29. Lakka TA, Venalainen JM, Rauramaa R, Salonen R, Tuomilehto J, Salonen JT. Relation of leisure-time physical activity and cardiorespiratory fitness to the risk of acute myocardial infarction. N Engl J Med. 1994; 330:1549–54.30. Wasenius N, Venojarvi M, Manderoos S, Surakka J, Lindholm H, Heinonen OJ, et al. Unfavorable influence of structured exercise program on total leisure-time physical activity. Scand J Med Sci Sports. 2014; 24:404–13.31. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998; 15:539–53.32. Eriksson JG, Forsen T, Tuomilehto J, Winter PD, Osmond C, Barker DJ. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. BMJ. 1999; 318:427–31.33. Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006; 38:904–9.
Article34. Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS Genet. 2006; 2:e190.
Article35. Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharmacol Ther. 2012; 136:82–93.
Article36. Kullmann S, Heni M, Hallschmid M, Fritsche A, Preissl H, Haring HU. Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol Rev. 2016; 96:1169–209.
Article37. Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000; 289:2122–5.
Article38. Qu J, Ko CW, Tso P, Bhargava A. Apolipoprotein A-IV: a multifunctional protein involved in protection against atherosclerosis and diabetes. Cells. 2019; 8:319.
Article39. Morita SY. Metabolism and modification of apolipoprotein Bcontaining lipoproteins involved in dyslipidemia and atherosclerosis. Biol Pharm Bull. 2016; 39:1–24.
Article40. Borai A, Livingstone C, Zarif H, Ferns G. Serum insulin-like growth factor binding protein-1: an improvement over other simple indices of insulin sensitivity in the assessment of subjects with normal glucose tolerance. Ann Clin Biochem. 2009; 46(Pt 2):109–13.
Article41. Mogul HR, Marshall M, Frey M, Burke HB, Wynn PS, Wilker S, et al. Insulin like growth factor-binding protein-1 as a marker for hyperinsulinemia in obese menopausal women. J Clin Endocrinol Metab. 1996; 81:4492–5.
Article42. Duarte AI, Santos MS, Oliveira CR, Moreira PI. Brain insulin signalling, glucose metabolism and females’ reproductive aging: a dangerous triad in Alzheimer’s disease. Neuropharmacology. 2018; 136(Pt B):223–42.
Article43. Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003; 88:2404–11.
Article44. Rettberg JR, Yao J, Brinton RD. Estrogen: a master regulator of bioenergetic systems in the brain and body. Front Neuroendocrinol. 2014; 35:8–30.
Article45. Basu A, Dube S, Basu R. Men are from Mars, women are from Venus: sex differences in insulin action and secretion. Adv Exp Med Biol. 2017; 1043:53–64.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Polygenic Risk Score and Precision Medicine in Diabetes
- Insulin resistance on receptor and post-receptor phases in streptozotocin-induced diabetes rats
- Insulin Resistance in Polycystic Ovary Syndrome
- Metabolically Healthy and Unhealthy Normal Weight and Obesity
- Insulin Secretion and Insulin Sensitivity in Women with a Previous Gestational Diabetes: Understanding of Pathogenesis of Type 2 Diabetes