J Korean Med Sci.
2024 Jul;39(28):e205. 10.3346/jkms.2024.39.e205.
Polypharmacy and Elevated Risk of Severe Adverse Events in Older Adults Based on the Korea Institute of Drug Safety and Risk Management-Korea Adverse Event Reporting System Database
- Affiliations
-
- 1Big Data Research Center, Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea
- 2Clinical Research Center, Asan Institute for Life Sciences, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Biomedical Engineering, Asan Medical Institute of Convergence Science and Technology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 4Healthcare Contents, EVIDNET, Seoul, Korea
- 5College of Medicine, Chungbuk National University, Cheongju, Korea
- 6Department of Computer Engineering, Gachon University, Seongnam, Korea
- 7Division of Biomedical Informatics, Seoul National University, Seoul, Korea
- 8Department of Information Medicine, Asan Medical Center, Seoul, Korea
- 9Department of Biomedical Informatics, Ulsan University College of Medicine, Ulsan, Korea
- KMID: 2558243
- DOI: http://doi.org/10.3346/jkms.2024.39.e205
Abstract
- Background
Older adults are at a higher risk of severe adverse drug events (ADEs) because of multimorbidity, polypharmacy, and lower physiological function. This study aimed to determine whether polypharmacy, defined as the use of ≥ 5 active drug ingredients, was associated with severe ADEs in this population.
Methods
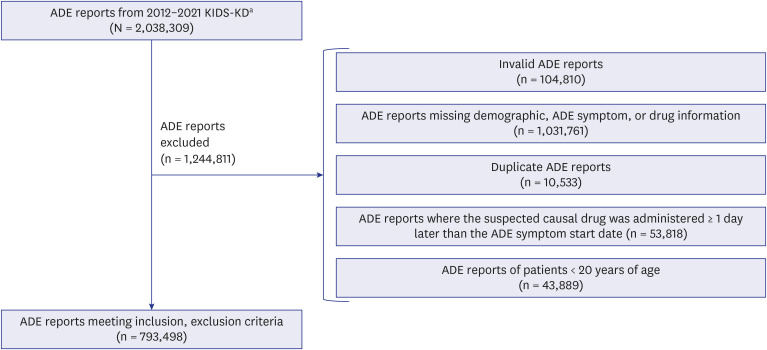
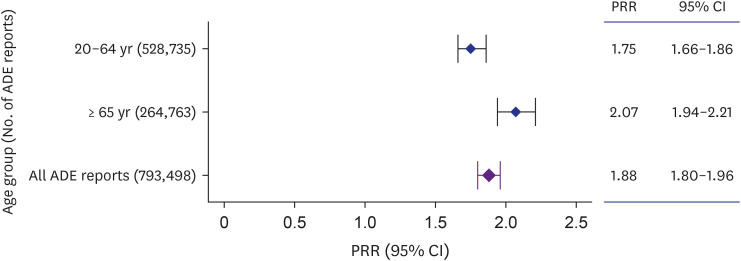
We used ADE reports from the Korea Institute of Drug Safety and Risk Management-Korea Adverse Event Reporting System Database, a national spontaneous ADE report system, from 2012 to 2021 to examine and compare the strength of association between polypharmacy and severe ADEs in older adults (≥ 65 years) and younger adults (20–64 years) using disproportionality analysis.
Results
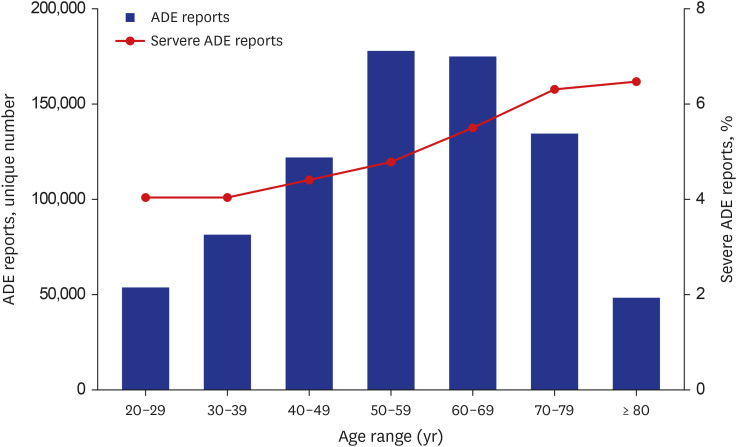
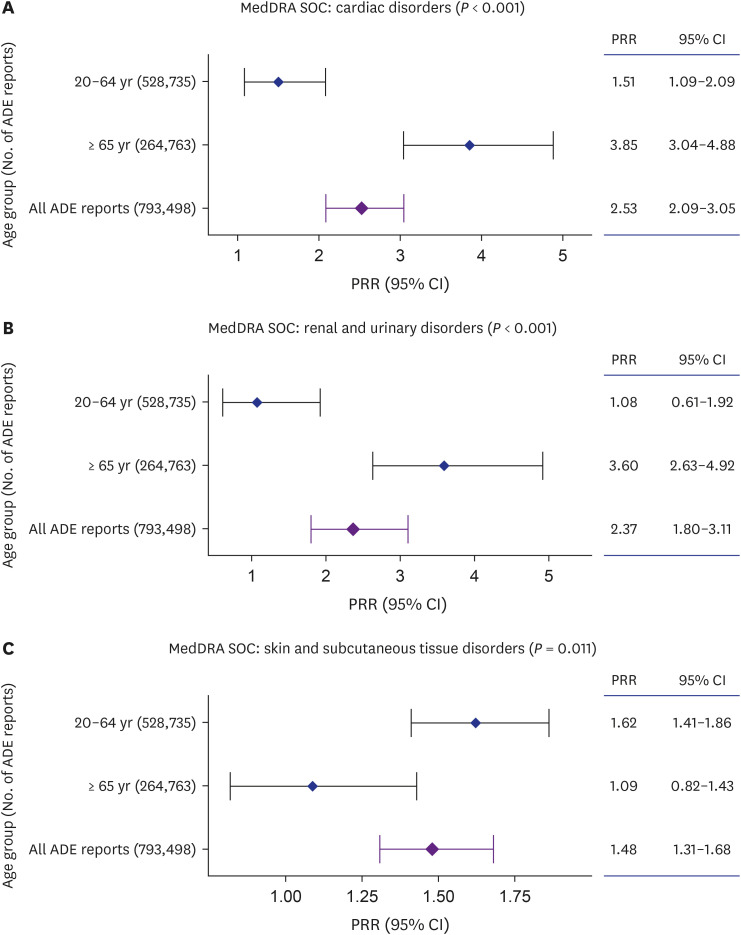
We found a significant association between severe ADEs of cardiac and renal/ urinary Medical Dictionary for Regulatory Activities System Organ Classes (MedDRA SOC) with polypharmacy in older adults. Regarding individual-level ADEs included in these MedDRA SOCs, acute cardiac arrest and renal failure were more significantly associated with polypharmacy in older adults compared with younger adults.
Conclusion
The addition of new drugs to the regimens of older adults warrants close monitoring of renal and cardiac symptoms.
Figure
Reference
-
1. World Health Organization. Ageing and health. Updated 2022. Accessed November 24, 2023. https://www.who.int/news-room/fact-sheets/detail/ageing-and-health .2. OECD. Health at a Glance 2023. Paris, France: OECD;2023.3. Bakerjian D. Overview of geriatric care. Updated 2020. Accessed March 26, 2020. https://www.merckmanuals.com/professional/geriatrics/provision-of-care-to-the-elderly/overview-of-geriatric-care .4. Vespa J, Medina L, Armstrong DM. 2020; 25–1144.5. Boersma P, Black LI, Ward BW. Prevalence of multiple chronic conditions among US adults, 2018. Prev Chronic Dis. 2020; 17:E106. PMID: 32945769.6. Remelli F, Ceresini MG, Trevisan C, Noale M, Volpato S. Prevalence and impact of polypharmacy in older patients with type 2 diabetes. Aging Clin Exp Res. 2022; 34(9):1969–1983. PMID: 35723858.7. Aggarwal P, Woolford SJ, Patel HP. Multi-morbidity and polypharmacy in older people: challenges and opportunities for clinical practice. Geriatrics (Basel). 2020; 5(4):85. PMID: 33126470.8. Fialová D, Kummer I, Držaić M, Leppee M. Ageism in medication use in older patients. Ayalon L, Tesch-Römer C, editors. Contemporary Perspectives on Ageism. Cham, Switzerland: Springer International Publishing;2018. p. 213–240.9. Osanlou R, Walker L, Hughes DA, Burnside G, Pirmohamed M. Adverse drug reactions, multimorbidity and polypharmacy: a prospective analysis of 1 month of medical admissions. BMJ Open. 2022; 12(7):e055551.10. Kardas P, Mair A, Stewart D, Lewek P. Optimizing polypharmacy management in the elderly: a comprehensive European benchmarking survey and the development of an innovative online benchmarking application. Front Pharmacol. 2023; 14:1254912. PMID: 37915419.11. Nightingale G, Mohamed MR, Holmes HM, Sharma M, Ramsdale E, Lu-Yao G, et al. Research priorities to address polypharmacy in older adults with cancer. J Geriatr Oncol. 2021; 12(6):964–970. PMID: 33589379.12. Lau SR, Waldorff F, Holm A, Frølich A, Andersen JS, Sallerup M, et al. Disentangling concepts of inappropriate polypharmacy in old age: a scoping review. BMC Public Health. 2023; 23(1):245. PMID: 36739368.13. Kabue S, Liu V, Dyer W, Raebel M, Nichols G, Schmittdiel J. Identifying common predictors of multiple adverse outcomes among elderly adults with type-2 diabetes. Med Care. 2019; 57(9):702–709. PMID: 31356411.14. Ramos LR, Tavares NU, Bertoldi AD, Farias MR, Oliveira MA, Luiza VL, et al. Polypharmacy and polymorbidity in older adults in Brazil: a public health challenge. Rev Saude Publica. 2016; 50(Suppl 2):9s.15. Cho HJ, Chae J, Yoon SH, Kim DS. Aging and the prevalence of polypharmacy and hyper-polypharmacy among older adults in South Korea: a national retrospective study during 2010–2019. Front Pharmacol. 2022; 13:866318. PMID: 35614938.16. Shimada K, Hasegawa S, Nakao S, Mukai R, Sasaoka S, Ueda N, et al. Adverse reaction profiles of hemorrhagic adverse reactions caused by direct oral anticoagulants analyzed using the Food and Drug Administration Adverse Event Reporting System (FAERS) database and the Japanese Adverse Drug Event Report (JADER) database. Int J Med Sci. 2019; 16(9):1295–1303. PMID: 31588196.17. Cho HJ, Chae J, Yoon SH, Kim DS. Factors related to polypharmacy and hyper-polypharmacy for the elderly: a nationwide cohort study using National Health Insurance data in South Korea. Clin Transl Sci. 2023; 16(2):193–205. PMID: 36401587.18. Korean Institute of Drug Safety and Risk Management. Korean Adverse Event Reporting System Database User Manual. 12nd ed. Anyang, Korea: Korea Institute of Drug Safety & Risk Management;2022.19. Drug Safety. Updated 2022. Accessed September 14, 2022. https://nedrug.mfds.go.kr/pbp/CCBGA01/getItem?infoName=%EC%84%B1%EB%B6%84&totalPages=8&limit=10&searchYn=true&page=1&&openDataInfoSeq=42 .20. Mabuchi T, Hosomi K, Yokoyama S, Takada M. Polypharmacy in elderly patients in Japan: analysis of Japanese real-world databases. J Clin Pharm Ther. 2020; 45(5):991–996. PMID: 31986233.21. Díez-Manglano J, Giménez-López M, Garcés-Horna V, Sevil-Puras M, Castellar-Otín E, González-García P, et al. Excessive polypharmacy and survival in polypathological patients. Eur J Clin Pharmacol. 2015; 71(6):733–739. PMID: 25911439.22. Jódar-Sánchez F, Malet-Larrea A, Martín JJ, García-Mochón L, López Del Amo MP, Martínez-Martínez F, et al. Cost-utility analysis of a medication review with follow-up service for older adults with polypharmacy in community pharmacies in Spain: the conSIGUE program. Pharmacoeconomics. 2015; 33(6):599–610. PMID: 25774017.23. Yang M, Lu J, Hao Q, Luo L, Dong B. Does residing in urban or rural areas affect the incidence of polypharmacy among older adults in western China? Arch Gerontol Geriatr. 2015; 60(2):328–333. PMID: 25440757.24. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna, Austria: R Foundation;2023.25. Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013; 382(9894):769–779. PMID: 23726390.26. Charlot M, Grove EL, Hansen PR, Olesen JB, Ahlehoff O, Selmer C, et al. Proton pump inhibitor use and risk of adverse cardiovascular events in aspirin treated patients with first time myocardial infarction: nationwide propensity score matched study. BMJ. 2011; 342:d2690. PMID: 21562004.27. Ozenberger K, Alexander GC, Shin JI, Whitsel EA, Qato DM. Use of prescription medications with cardiovascular adverse effects among older adults in the United States. Pharmacoepidemiol Drug Saf. 2022; 31(10):1027–1038. PMID: 35569118.28. Formica M, Politano P, Marazzi F, Tamagnone M, Serra I, Marengo M, et al. Acute kidney injury and chronic kidney disease in the elderly and polypharmacy. Blood Purif. 2018; 46(4):332–336. PMID: 30176676.29. Ernst R, Fischer K, de Godoi Rezende Costa Molino C, Orav EJ, Theiler R, Meyer U, et al. Polypharmacy and kidney function in community-dwelling adults age 60 years and older: a prospective observational study. J Am Med Dir Assoc. 2020; 21(2):254–259.e1. PMID: 31501003.30. Chao CT, Tsai HB, Wu CY, Lin YF, Hsu NC, Chen JS, et al. Cumulative cardiovascular polypharmacy is associated with the risk of acute kidney injury in elderly patients. Medicine (Baltimore). 2015; 94(31):e1251. PMID: 26252287.31. Seo DE, Kim S, Park BJ. Signals of adverse drug reactions of paliperidone compared to other atypical antipsychotics using the Korean adverse event reporting system database. Clin Drug Investig. 2020; 40(9):873–881.32. Baek YH, Kim HJ, Bae JH, Lee H, Oh IS, Kim WJ, et al. Benzodiazepine-related cognitive impairment or dementia: a signal detection study using a case/non-case approach. Psychiatry Investig. 2020; 17(6):587–595.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Class-Effect Study of Vaccine Signal Detection Using Korea Adverse Event Reporting System Database
- Signal Detection and Safety Information Generation of Aripiprazole in Spontaneous Adverse Event Reports Database
- Signal Detection of Adverse Event of Metoclopramide in Korea Adverse Event Reporting System (KAERS)
- Comprehensive management of polypharmacy in older patients with diabetes
- Adverse Events Related to Zolpidem in the Korea Pharmaceutical Association Adverse Event Reporting System