Clin Endosc.
2024 Jul;57(4):417-423. 10.5946/ce.2023.279.
Potassium-competitive acid blocker-associated gastric mucosal lesions
- Affiliations
-
- 1Department of Gastroenterology, National Hospital Organization Hakodate National Hospital, Hakodate, Japan
- 2Department of Pathology, National Hospital Organization Hakodate National Hospital, Hakodate, Japan
- 3Hokkaido Cancer Society, Sapporo, Japan
- KMID: 2558095
- DOI: http://doi.org/10.5946/ce.2023.279
Abstract
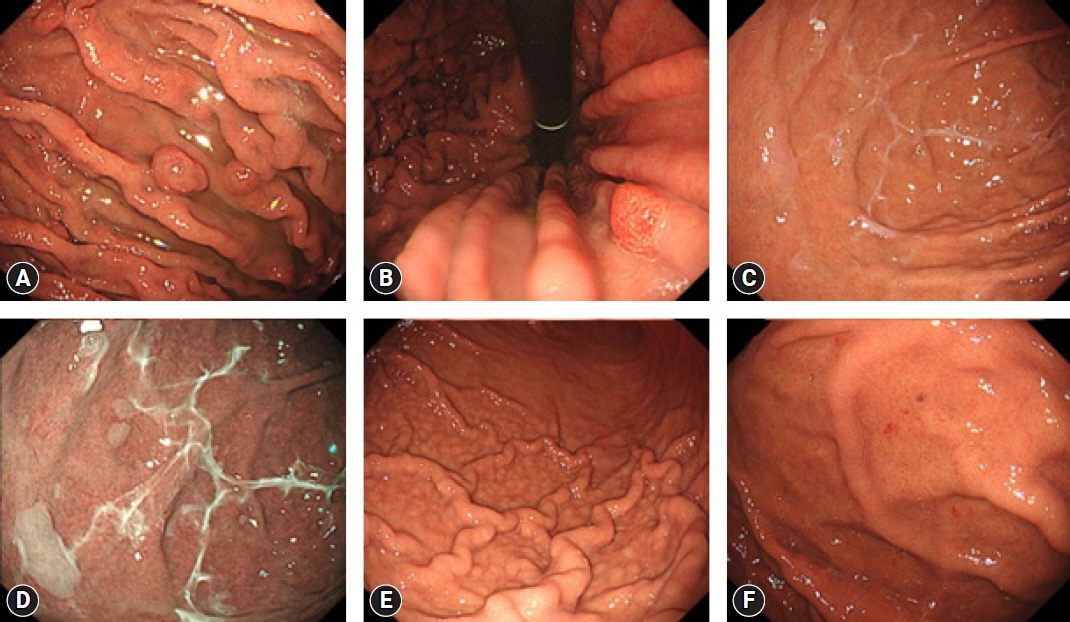
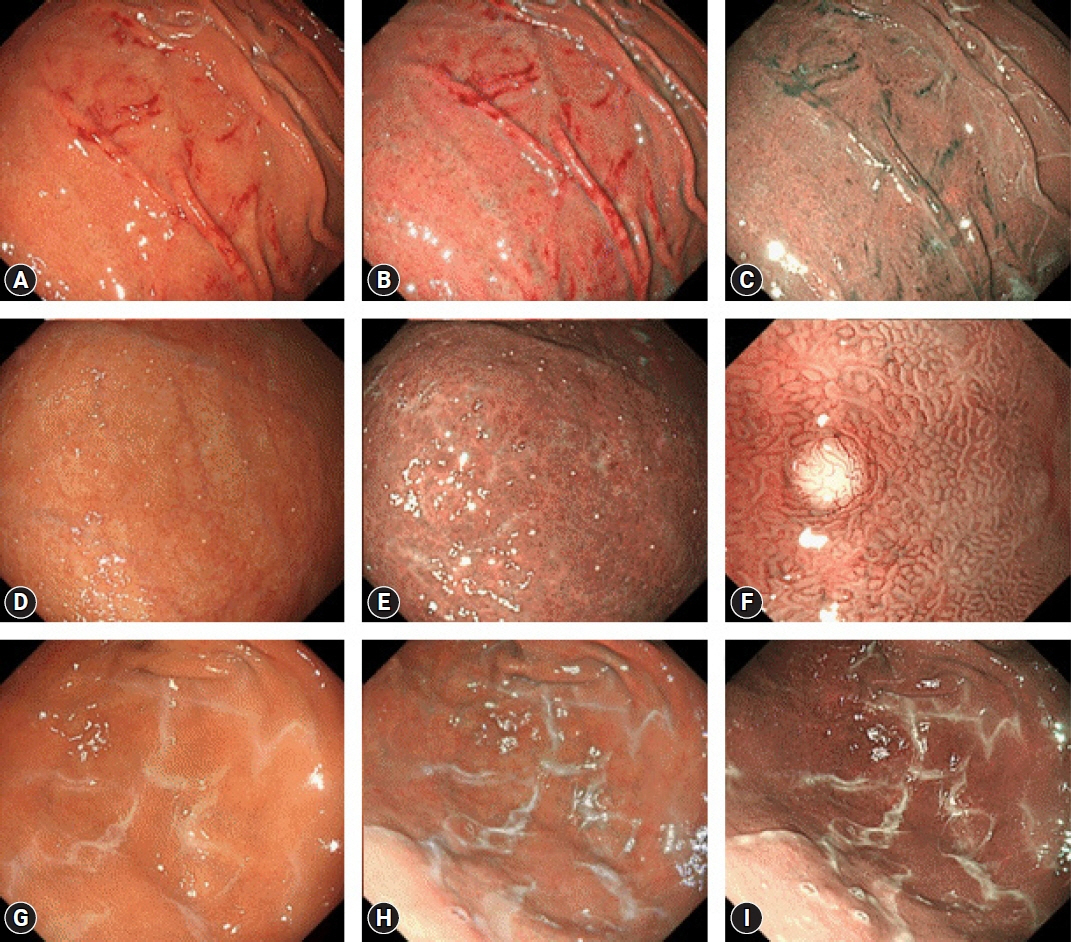
- Since the introduction of vonoprazan, a potassium-competitive acid blocker (P-CAB), it has been demonstrated to reversibly inhibit gastric acid secretion by engaging in potassium-competitive ionic binding to H+/K+-ATPase. In contrast, proton pump inhibitors (PPIs) achieve H+/K+-ATPase inhibition through covalent binding to cysteine residues of the proton pump. Reported cases have indicated an emerging trend of P-CAB-related gastropathies, similar to those associated with PPIs, as well as unique gastropathies specific to P-CAB use, such as the identification of web-like mucus. Pathologically, parietal cell profusions, which show a positively correlated with hypergastrinemia, have a higher incidence in P-CAB users compared to PPI users. Thus, this review aims to summarize the endoscopic and pathological findings reported to date concerning P-CAB-related gastric mucosal lesions. Additionally, it seeks to discuss the differences between the PPIs and P-CABs in terms of the formation and frequency of associated gastropathies. This review highlights the evident differences in the mechanism of action and potency of acid inhibition between P-CABs and PPIs, notably contributing to differences in the formation and frequency of associated gastropathies. It emphasizes the necessity to distinguish between P-CAB-related and PPI-related gastropathies in the clinical setting.
Keyword
Figure
Reference
-
1. Strand DS, Kim D, Peura DA. 25 Years of proton pump inhibitors: a comprehensive review. Gut Liver. 2017; 11:27–37.
Article2. Arikawa Y, Nishida H, Kurasawa O, et al. Discovery of a novel pyrrole derivative 1-[5-(2-fluorophenyl)-1-(pyrid in-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine fumarate (TAK-438) as a potassium-competitive acid blocker (P-CAB). J Med Chem. 2012; 55:4446–4456.
Article3. Parsons ME, Keeling DJ. Novel approaches to the pharmacological blockade of gastric acid secretion. Expert Opin Investig Drugs. 2005; 14:411–421.
Article4. Adachi K, Katsube T, Kawamura A, et al. CYP2C19 genotype status and intragastric pH during dosing with lansoprazole or rabeprazole. Aliment Pharmacol Ther. 2000; 14:1259–1266.
Article5. Sakurai Y, Mori Y, Okamoto H, et al. Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects: a randomised open-label cross-over study. Aliment Pharmacol Ther. 2015; 42:719–730.
Article6. Jansen JB, Klinkenberg-Knol EC, Meuwissen SG, et al. Effect of long-term treatment with omeprazole on serum gastrin and serum group A and C pepsinogens in patients with reflux esophagitis. Gastroenterology. 1990; 99:621–628.
Article7. Kojima Y, Takeuchi T, Sanomura M, et al. Does the novel potassium-competitive acid blocker vonoprazan cause more hypergastrinemia than conventional proton pump inhibitors?: a multicenter prospective cross-sectional study. Digestion. 2018; 97:70–75.
Article8. Wang TC, Koh TJ, Varro A, et al. Processing and proliferative effects of human progastrin in transgenic mice. J Clin Invest. 1996; 98:1918–1929.
Article9. Cats A, Schenk BE, Bloemena E, et al. Parietal cell protrusions and fundic gland cysts during omeprazole maintenance treatment. Hum Pathol. 2000; 31:684–690.
Article10. Kumar KR, Iqbal R, Coss E, et al. Helicobacter gastritis induces changes in the oxyntic mucosa indistinguishable from the effects of proton pump inhibitors. Hum Pathol. 2013; 44:2706–2710.
Article11. Stolte M, Bethke B, Seifert E, et al. Observation of gastric glandular cysts in the corpus mucosa of the stomach under omeprazole treatment. Z Gastroenterol. 1995; 33:146–149.12. Declich P, Ambrosiani L, Bellone S, et al. Parietal cell hyperplasia with deep cystic dilations: a lesion closely mimicking fundic gland polyps. Am J Gastroenterol. 2000; 95:566–568.
Article13. Kim GH. Proton pump inhibitor-related gastric mucosal changes. Gut Liver. 2021; 15:646–652.
Article14. Martin FC, Chenevix-Trench G, Yeomans ND. Systematic review with meta-analysis: fundic gland polyps and proton pump inhibitors. Aliment Pharmacol Ther. 2016; 44:915–925.
Article15. Iwamuro M, Shiraha H, Okada H. Gastric polyps’ regression after potassium-competitive acid blocker cessation. J Gen Fam Med. 2022; 23:358–359.
Article16. Nishimura N, Mizuno M, Matsueda K. Gastroduodenal intussusception due to vonoprazan-induced gastric polyps. Intern Med. 2022; 61:1305–1306.
Article17. Haruma K, Kinoshita Y, Yao T, et al. Randomised clinical trial: 3-year interim analysis results of the VISION trial to evaluate the long-term safety of vonoprazan as maintenance treatment in patients with erosive oesophagitis. BMC Gastroenterol. 2023; 23:139.
Article18. Shinozaki S, Osawa H, Hayashi Y, et al. Changes in gastric morphology during long-term use of vonoprazan compared to proton pump inhibitors. Singapore Med J. 2022; 63:283–287.
Article19. Hongo M, Fujimoto K; Gastric Polyps Study Group. Incidence and risk factor of fundic gland polyp and hyperplastic polyp in long-term proton pump inhibitor therapy: a prospective study in Japan. J Gastroenterol. 2010; 45:618–624.
Article20. Goto C, Okimoto K, Matsusaka K, et al. Long-term vonoprazan administration causes gastric fundic gland-type hyperplastic polyps and chronic bleeding. Clin J Gastroenterol. 2023; 16:159–163.
Article21. Hatano Y, Haruma K, Kamada T, et al. Factors associated with gastric black spot, white flat elevated mucosa, and cobblestone-like mucosa: a cross-sectional study. Digestion. 2018; 98:185–193.
Article22. Uedo N, Yamaoka R, Yao K. Multiple white flat lesions in the gastric corpus are not intestinal metaplasia. Endoscopy. 2017; 49:615–616.
Article23. Shinozaki S, Osawa H, Miura Y, et al. The effect of proton pump inhibitors and vonoprazan on the development of ‘gastric mucosal redness’. Biomed Rep. 2022; 16:51.
Article24. Miyamoto S, Kudo T, Kato M, et al. Endoscopic ultrasonography features of gastric mucosal cobblestone-like changes from a proton-pump inhibitor. Clin J Gastroenterol. 2017; 10:220–223.
Article25. Miyamoto S, Matsuno Y, Kato M, et al. Parietal cell protrusions and dilated oxyntic glands from use of vonoprazan. Am J Gastroenterol. 2017; 112:1899–1901.
Article26. Hatano Y, Haruma K, Ayaki M, et al. Black spot, a novel gastric finding potentially induced by proton pump inhibitors. Intern Med. 2016; 55:3079–3084.
Article27. Kiso M, Ito M, Boda T, et al. Endoscopic findings of the gastric mucosa during long-term use of proton pump inhibitor - a multicenter study. Scand J Gastroenterol. 2017; 52:828–832.
Article28. Kubo K, Kimura N, Matsuda S, et al. Vonoprazan-associated gastric mucosal redness: a report of four cases. Intern Med. 2020; 59:507–511.
Article29. Kubo K, Kimura N, Watanabe R, et al. Vonoprazan-associated gastric mucosal redness in non-Helicobacter pylori-infected and Helicobacter pylori-eradicated stomach. Case Rep Gastroenterol. 2021; 15:751–758.
Article30. Kinoshita Y, Yamasaki T, Saruta M. Numerous white nodules during continuous administration of a potent gastric acid suppressor. Gastrointest Endosc. 2021; 93:766.
Article31. Nishiyama N, Kobara H, Kagawa S, et al. Vonoprazan may cause white globe appearance in nonatrophic mucosa of stomach. Gastrointest Endosc. 2021; 93:767–768.
Article32. Yoshizaki T, Morisawa T, Fujinami M, et al. Propensity score matching analysis: incidence and risk factors for “stardust” gastric mucosa, a novel gastric finding potentially induced by vonoprazan. Aliment Pharmacol Ther. 2021; 53:94–102.
Article33. Nishiyama N, Kobara H, Ayaki M, et al. White spot, a novel endoscopic finding, may be associated with acid-suppressing agents and hypergastrinemia. J Clin Med. 2021; 10:2625.
Article34. Iwamuro M, Tanaka T, Sakae H, et al. Two cases of white globe appearance in non-cancerous stomach. Ecancermedicalscience. 2018; 12:856.
Article35. Miwa W, Hiratsuka T, Sato K, et al. Development of white globe appearance lesions in the noncancerous stomach after vonoprazan administration: a report of two cases with a literature review. Clin J Gastroenterol. 2021; 14:48–58.
Article36. Miwa W, Hiratsuka T, Sato K, et al. Marked reduction in the number of white globe appearance lesions in the noncancerous stomach after exchanging vonoprazan for esomeprazole treatment: a follow-up case report. Clin J Gastroenterol. 2021; 14:1046–1051.
Article37. Haruma K, Kato M, Inoue K, et al. Kyoto classification of gastritis. 3rd ed. Nihon Medical Center;2023.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long-term follow-up of patients developing gastric mucosal lesions after initiating the potassium-competitive acid blocker vonoprazan
- Role of Acid Suppression in Acid-related Diseases: Proton Pump Inhibitor and Potassium-competitive Acid Blocker
- Treatment of Acid-related Diseases Using Potassium-competitive Acid Blockers
- Role of Potassium-competitive Acid Blockers in the Treatment of Gastric Acid-related Disorders
- Optimal Use of Proton Pump Inhibitors and Potassium-competitive Acid Blockers