Diabetes Metab J.
2024 Jul;48(4):740-751. 10.4093/dmj.2023.0189.
A Composite Blood Biomarker Including AKR1B10 and Cytokeratin 18 for Progressive Types of Nonalcoholic Fatty Liver Disease
- Affiliations
-
- 1Department of Radiology, Gachon University Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- 2Department of Family Medicine, Gachon University Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- 3Department of Surgery, Gachon University Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- 4Department of Internal Medicine, Gachon University Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- 5Department of Biomedical Engineering, Gachon University Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- KMID: 2558041
- DOI: http://doi.org/10.4093/dmj.2023.0189
Abstract
- Background
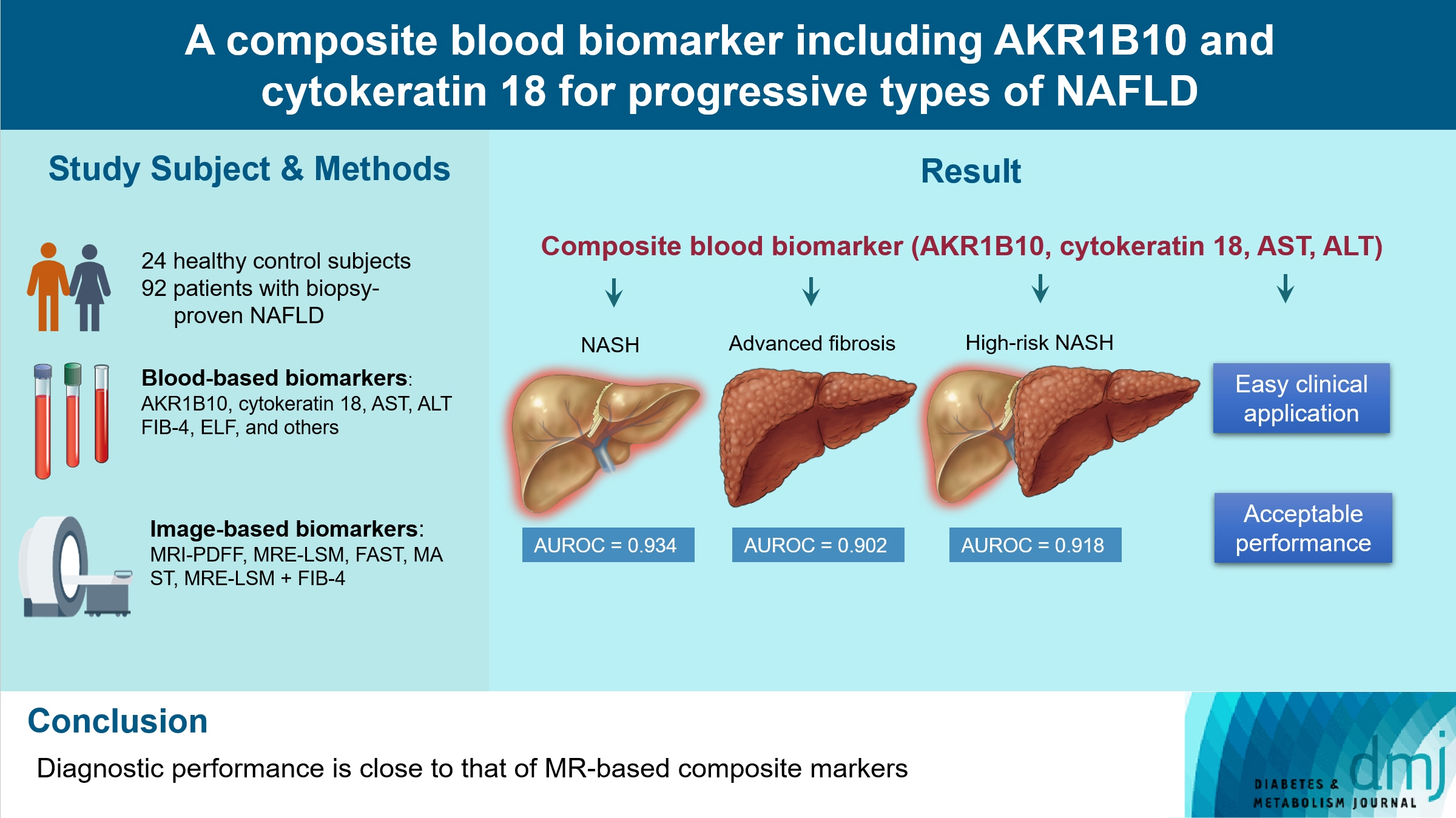
We aimed to evaluate whether composite blood biomarkers including aldo-keto reductase family 1 member B10 (AKR1B10) and cytokeratin 18 (CK-18; a nonalcoholic steatohepatitis [NASH] marker) have clinically applicable performance for the diagnosis of NASH, advanced liver fibrosis, and high-risk NASH (NASH+significant fibrosis).
Methods
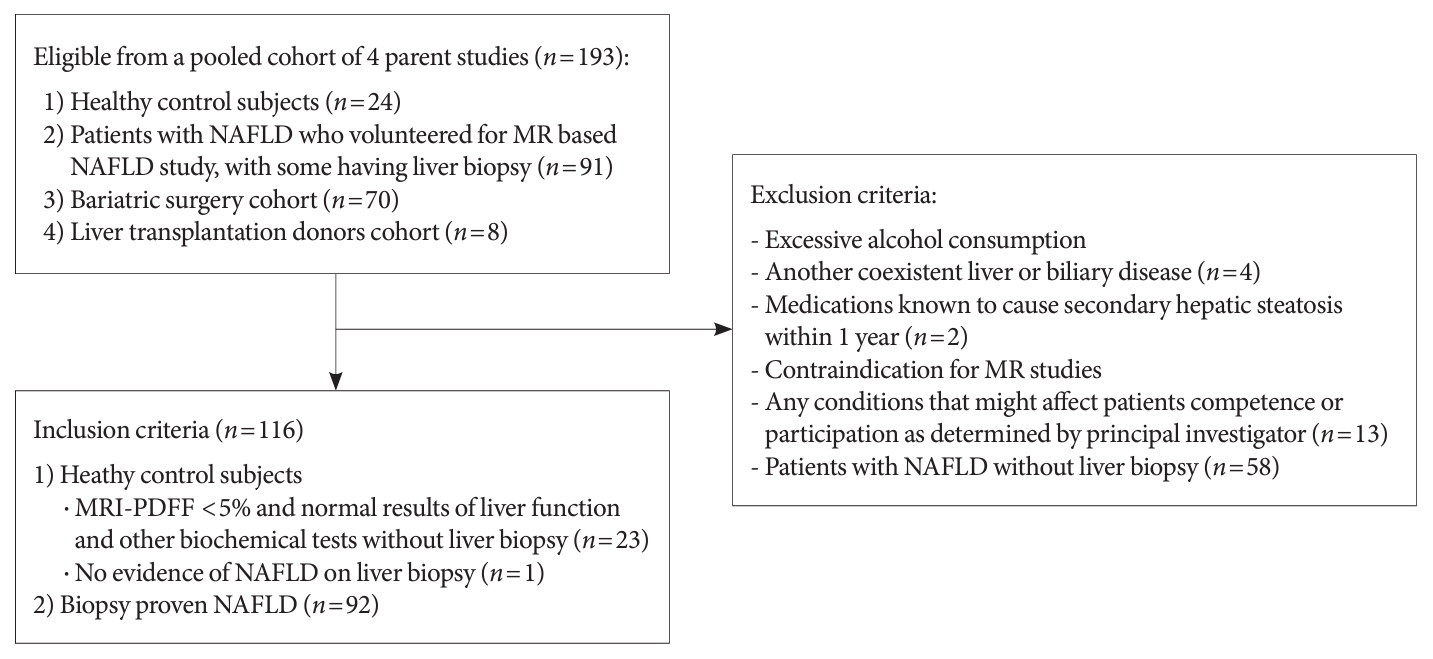
A total of 116 subjects including healthy control subjects and patients with biopsy-proven nonalcoholic fatty liver disease (NAFLD) were analyzed to assess composite blood-based and imaging-based biomarkers either singly or in combination.
Results
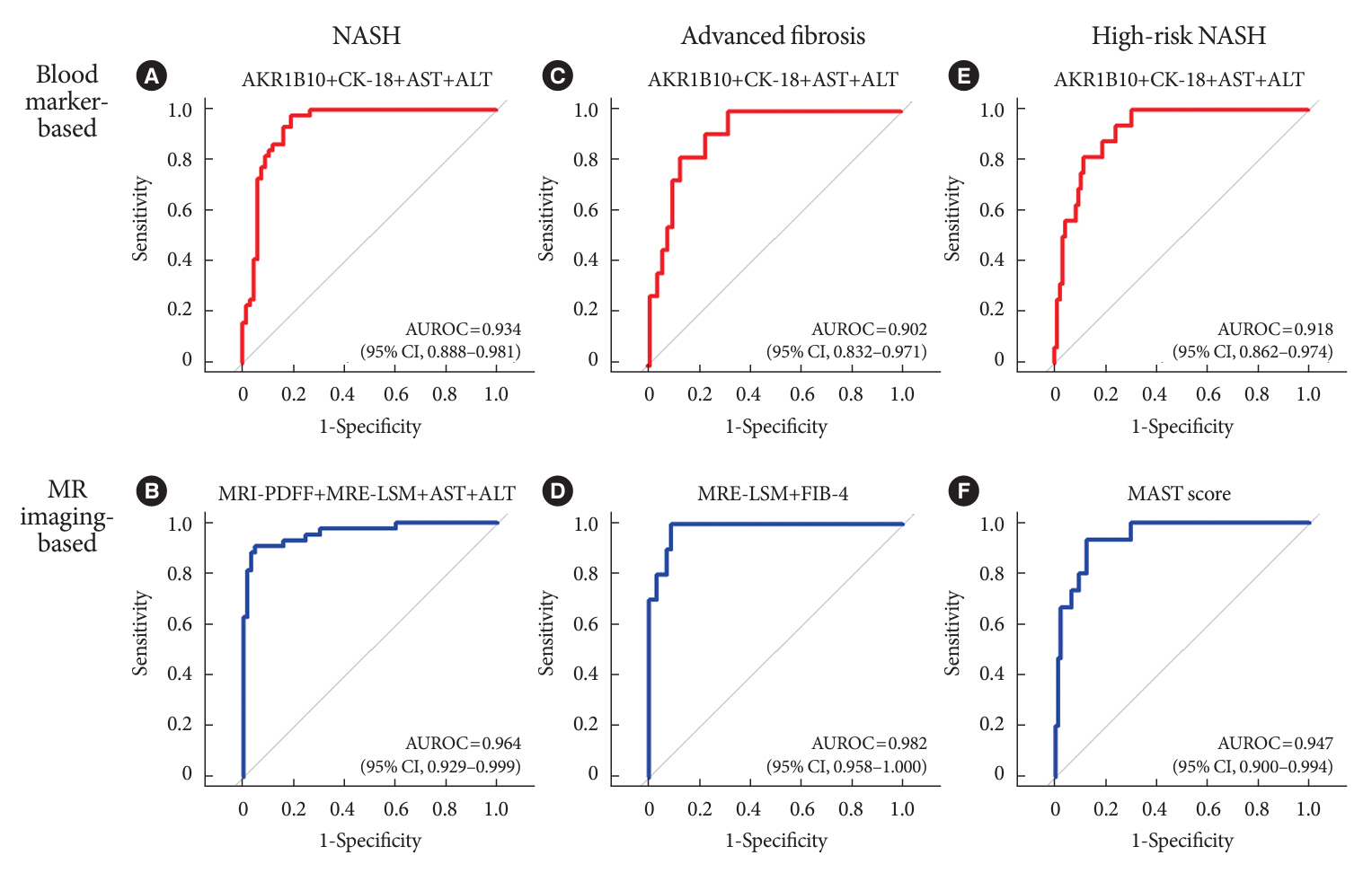
A composite blood biomarker comprised of AKR1B10, CK-18, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) showed excellent performance for the diagnosis of, NASH, advanced fibrosis, and high-risk NASH, with area under the receiver operating characteristic curve values of 0.934 (95% confidence interval [CI], 0.888 to 0.981), 0.902 (95% CI, 0.832 to 0.971), and 0.918 (95% CI, 0.862 to 0.974), respectively. However, the performance of this blood composite biomarker was inferior to that various magnetic resonance (MR)-based composite biomarkers, such as proton density fat fraction/MR elastography- liver stiffness measurement (MRE-LSM)/ALT/AST for NASH, MRE-LSM+fibrosis-4 index for advanced fibrosis, and the known MR imaging-AST (MAST) score for high-risk NASH.
Conclusion
Our blood composite biomarker can be useful to distinguish progressive forms of NAFLD as an initial noninvasive test when MR-based tools are not available.
Keyword
Figure
Reference
-
1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease: meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016; 64:73–84.
Article2. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018; 67:328–57.3. Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology. 2020; 158:1851–64.
Article4. Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, et al. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol. 2018; 113:1649–59.
Article5. Sanyal AJ, Van Natta ML, Clark J, Neuschwander-Tetri BA, Diehl A, Dasarathy S, et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med. 2021; 385:1559–69.
Article6. Rinella ME, Tacke F, Sanyal AJ, Anstee QM; participants of the AASLD/EASL Workshop. Report on the AASLD/EASL joint workshop on clinical trial endpoints in NAFLD. J Hepatol. 2019; 71:823–33.
Article7. Allen AM, Therneau TM, Ahmed OT, Gidener T, Mara KC, Larson JJ, et al. Clinical course of non-alcoholic fatty liver disease and the implications for clinical trial design. J Hepatol. 2022; 77:1237–45.8. Kim BK, Tamaki N, Imajo K, Yoneda M, Sutter N, Jung J, et al. Head-to-head comparison between MEFIB, MAST, and FAST for detecting stage 2 fibrosis or higher among patients with NAFLD. J Hepatol. 2022; 77:1482–90.
Article9. Friedman SL, Ratziu V, Harrison SA, Abdelmalek MF, Aithal GP, Caballeria J, et al. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 2018; 67:1754–67.
Article10. Lee DH. Noninvasive evaluation of nonalcoholic fatty liver disease. Endocrinol Metab (Seoul). 2020; 35:243–59.
Article11. Boyle M, Tiniakos D, Schattenberg JM, Ratziu V, Bugianessi E, Petta S, et al. Performance of the PRO-C3 collagen neo-epitope biomarker in non-alcoholic fatty liver disease. JHEP Rep. 2019; 1:188–98.
Article12. Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006; 44:27–33.13. Eguchi A, Iwasa M, Yamada M, Tamai Y, Shigefuku R, Hasegawa H, et al. A new detection system for serum fragmented cytokeratin 18 as a biomarker reflecting histologic activities of human nonalcoholic steatohepatitis. Hepatol Commun. 2022; 6:1987–99.
Article14. Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009; 50:1072–8.
Article15. Park A, Choi SJ, Park S, Kim SM, Lee HE, Joo M, et al. Plasma aldo-keto reductase family 1 member B10 as a biomarker performs well in the diagnosis of nonalcoholic steatohepatitis and fibrosis. Int J Mol Sci. 2022; 23:5035.
Article16. Govaere O, Cockell S, Tiniakos D, Queen R, Younes R, Vacca M, et al. Transcriptomic profiling across the nonalcoholic fatty liver disease spectrum reveals gene signatures for steatohepatitis and fibrosis. Sci Transl Med. 2020; 12:eaba4448.
Article17. Martin HJ, Breyer-Pfaff U, Wsol V, Venz S, Block S, Maser E. Purification and characterization of akr1b10 from human liver: role in carbonyl reduction of xenobiotics. Drug Metab Dispos. 2006; 34:464–70.18. Endo S, Matsunaga T, Nishinaka T. The role of AKR1B10 in physiology and pathophysiology. Metabolites. 2021; 11:332.
Article19. Kanno M, Kawaguchi K, Honda M, Horii R, Takatori H, Shimakami T, et al. Serum aldo-keto reductase family 1 member B10 predicts advanced liver fibrosis and fatal complications of nonalcoholic steatohepatitis. J Gastroenterol. 2019; 54:549–57.
Article20. DiStefano JK, Davis B. Diagnostic and prognostic potential of AKR1B10 in human hepatocellular carcinoma. Cancers (Basel). 2019; 11:486.
Article21. Luo D, Bu Y, Ma J, Rajput S, He Y, Cai G, et al. Heat shock protein 90-α mediates aldo-keto reductase 1B10 (AKR1B10) protein secretion through secretory lysosomes. J Biol Chem. 2013; 288:36733–40.
Article22. Abdelmegeed MA, Banerjee A, Yoo SH, Jang S, Gonzalez FJ, Song BJ. Critical role of cytochrome P450 2E1 (CYP2E1) in the development of high fat-induced non-alcoholic steatohepatitis. J Hepatol. 2012; 57:860–6.23. Dulai PS, Sirlin CB, Loomba R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: clinical trials to clinical practice. J Hepatol. 2016; 65:1006–16.
Article24. Andersson A, Kelly M, Imajo K, Nakajima A, Fallowfield JA, Hirschfield G, et al. Clinical utility of magnetic resonance imaging biomarkers for identifying nonalcoholic steatohepatitis patients at high risk of progression: a multicenter pooled data and meta-analysis. Clin Gastroenterol Hepatol. 2022; 20:2451–61.
Article25. Dennis A, Mouchti S, Kelly M, Fallowfield JA, Hirschfield G, Pavlides M, et al. A composite biomarker using multiparametric magnetic resonance imaging and blood analytes accurately identifies patients with non-alcoholic steatohepatitis and significant fibrosis. Sci Rep. 2020; 10:15308.
Article26. Choi SJ, Kim SM, Kim YS, Kwon OS, Shin SK, Kim KK, et al. Magnetic resonance-based assessments better capture pathophysiologic profiles and progression in nonalcoholic fatty liver disease. Diabetes Metab J. 2021; 45:739–52.
Article27. Newsome PN, Sasso M, Deeks JJ, Paredes A, Boursier J, Chan WK, et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020; 5:362–73.28. Jung J, Loomba RR, Imajo K, Madamba E, Gandhi S, Bettencourt R, et al. MRE combined with FIB-4 (MEFIB) index in detection of candidates for pharmacological treatment of NASH-related fibrosis. Gut. 2021; 70:1946–53.
Article29. Noureddin M, Truong E, Gornbein JA, Saouaf R, Guindi M, Todo T, et al. MRI-based (MAST) score accurately identifies patients with NASH and significant fibrosis. J Hepatol. 2022; 76:781–7.
Article30. Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007; 46:32–6.
Article31. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005; 41:1313–21.
Article32. Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001; 344:495–500.33. Davison BA, Harrison SA, Cotter G, Alkhouri N, Sanyal A, Edwards C, et al. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J Hepatol. 2020; 73:1322–32.
Article34. Lee BW, Lee YH, Park CY, Rhee EJ, Lee WY, Kim NH, et al. Non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus: a position statement of the Fatty Liver Research Group of the Korean Diabetes Association. Diabetes Metab J. 2020; 44:382–401.
Article35. Cusi K, Isaacs S, Barb D, Basu R, Caprio S, Garvey WT, et al. American Association of Clinical Endocrinology clinical practice guideline for the diagnosis and management of nonalcoholic fatty liver disease in primary care and endocrinology clinical settings: co-sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr Pract. 2022; 28:528–62.36. Harrison SA, Wong VW, Okanoue T, Bzowej N, Vuppalanchi R, Younes Z, et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: results from randomized phase III STELLAR trials. J Hepatol. 2020; 73:26–39.37. Banerjee R, Pavlides M, Tunnicliffe EM, Piechnik SK, Sarania N, Philips R, et al. Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J Hepatol. 2014; 60:69–77.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Noninvasive serum biomarkers for liver steatosis in nonalcoholic fatty liver disease: Current and future developments
- Elevated serum bilirubin levels are inversely associated with nonalcoholic fatty liver disease
- Nonalcoholic fatty liver disease: pathogenesis and treatment
- Pharmacological advances in the treatment of nonalcoholic fatty liver diseases : focused on global results of randomized controlled trials
- The crosstalk between insulin resistance and nonalcoholic fatty liver disease/metabolic dysfunction-associated fatty liver disease: a culprit or a consequence?