Ann Lab Med.
2024 Jul;44(4):359-362. 10.3343/alm.2023.0393.
Comparative Analysis of AB vs. ABO-specific Plasma for Desensitization in Blood Group O Recipients: An In Vitro Study
- Affiliations
-
- 1Department of Laboratory Medicine, Ajou University School of Medicine, Suwon, Korea
- KMID: 2557935
- DOI: http://doi.org/10.3343/alm.2023.0393
Abstract
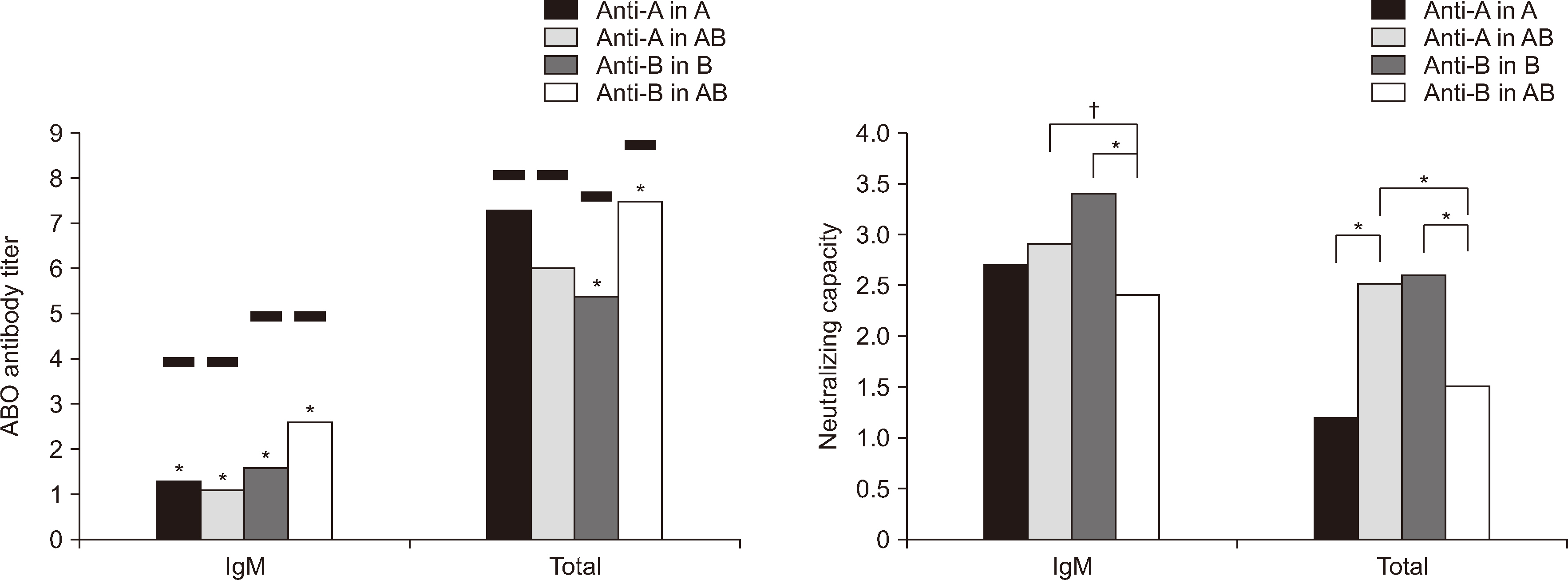
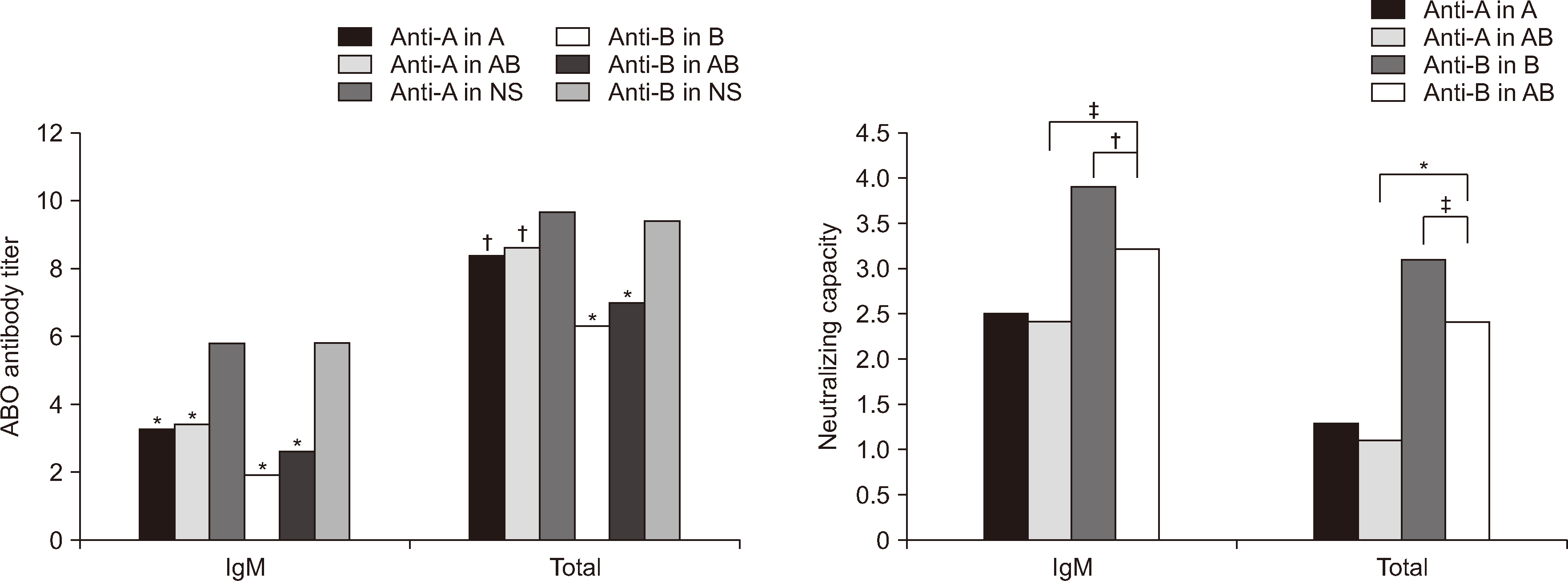
- Neutralizing capacity measurement (NCM) of soluble ABH substances (SAS) in plasma was assessed to guide the selection of the appropriate ABO group of fresh-frozen plasma (FFP) for plasma exchange (PE) in blood group O recipients with ABO-incompatible transplantations. Neutralizing capacity was assessed by measuring anti-A and/or anti-B titers in samples comprising one unit of O FFP and 10 O EDTA plasma samples and subtracting the binary logarithm of the titer in each group with a saline dilution. Ten EDTA plasma samples with Lewis b (Leb ) antigen positivity and 10 sets of pooled FFP from each blood group were used as diluents. In O FFP, the NCM values (mean ± SD) were 3.4 ± 0.52 (2.6 ± 0.52) and 2.6 ± 0.52 (1.5 ± 0.3) in B and AB for IgM (total antibody) anti-B (both P < 0.001), and in the 10 O EDTA plasma samples, they were 3.9 ± 0.88 (3.1 ± 0.88) and 3.2 ± 0.79 (2.4 ± 0.97) for IgM (P = 0.0013) and total anti-B (P = 0.025), respectively. In vitro analysis revealed that B FFP is more effective than AB FFP in reducing IgM and total anti-B antibody titers in O recipients, regardless of Le b antigen positivity.
Figure
Reference
-
References
1. Connelly-Smith L, Alquist CR, Aqui NA, Hofmann JC, Klingel R, Onwuemene OA, et al. 2023; Guidelines on the use of therapeutic apheresis in clinical practice - evidence-based approach from the writing committee of the American Society for Apheresis: the ninth special issue. J Clin Apher. 38:77–278. DOI: 10.1002/jca.22043. PMID: 37017433.
Article2. Parmentier SP, Rosenkranz E, Schirutschke H, Opgenoorth M, Quick C, Hoelig K, et al. 2017; Comparing the efficacy of three techniques to reduce isoagglutinin titers in ABO incompatible kidney transplant recipients. Atheroscler Suppl. 30:253–6. DOI: 10.1016/j.atherosclerosissup.2017.05.017. PMID: 29096846.3. Chung Y, Ko DH, Lim J, Kim KH, Kim H. 2022; Choice of ABO group for blood component transfusion in ABO-incompatible solid organ transplantation: a questionnaire survey in Korea and guideline proposal. Ann Lab Med. 42:105–9. DOI: 10.3343/alm.2022.42.1.105. PMID: 34374356. PMCID: PMC8368232.
Article4. Krog GR, Lorenzen H, Clausen FB, Hansen AT, Donneborg ML, Dziegiel MH. 2022; ABO haemolytic disease of the newborn: improved prediction by novel integration of causative and protective factors in newborn and mother. Vox Sang. 117:415–23. DOI: 10.1111/vox.13195. PMID: 34409614.5. Yang JJ, Ryu KS, Kim JS, Chung Y, Kim H, Hwang SH, et al. 2021; Evaluation of safety of using incompatible plasma for therapeutic plasma exchange during shortage of AB plasma. J Clin Apher. 36:628–33. DOI: 10.1002/jca.21904. PMID: 33950554.6. Won DI, Ham JY, Kim CD, Suh JS, Kim BC. 2015; Benefits of fresh-frozen plasma as a replacement fluid to neutralize ABO antibodies. J Clin Apher. 30:288–96. DOI: 10.1002/jca.21378. PMID: 25546477.7. Saboor M, Ullah A, Qamar K, Mir A, Monuddin . 2014; Frequency of ABH secretors and non secretors: a cross sectional study in Karachi. Pak J Med Sci. 30:189–93. DOI: 10.12669/pjms.301.4194. PMID: 24639859. PMCID: PMC3955570.8. Fung MK, Eder AF, Spitalnik SL, Westhoff CM. 2017. Technical manual. 19th ed. American Association of Blood Banks;Bethesda: p. Methods 2–8. DOI: 10.4160/9789290604808.9. Parigian MJ. 1995; An ELISA procedure for the detection of soluble ABH blood group substance in semen, saliva, and vaginal samples. J Forensic Sci. 40:122–5. DOI: 10.1520/JFS13774J. PMID: 7876793.
Article10. Zhou B, Guo JY, Wang CX, Chen J. 1990; The rapid determination of the ABO group from body fluids (or stains) by dot enzyme-linked immunosorbent assay (dot-ELISA) using enzyme-labeled monoclonal antibodies. J Forensic Sci. 35:1125–32. DOI: 10.1520/JFS12934J. PMID: 2230686.
Article11. Song SU, An SS, Ryu SW, Kim JS, Suh IB. 2008; Evaluation of the genotypes of the Lewis blood group in a Korean population using direct sequencing. Korean J Hematol. 43:34–42. DOI: 10.5045/kjh.2008.43.1.34.
Article12. Jaff MS. 2010; Higher frequency of secretor phenotype in O blood group - its benefits in prevention and/or treatment of some diseases. Int J Nanomedicine. 5:901–5. DOI: 10.2147/IJN.S13980. PMID: 21116330. PMCID: PMC2990383.13. Rajawat G, Ramalingam K, Pareek R, Singh G, Narula H, Aggarwal A. 2023; Assessment of salivary ABO blood group antigens and secretor status in Srinagar, Rajasthan: a correlational analysis of 300 samples. Cureus. 15:e37415. DOI: 10.7759/cureus.37415. PMID: 37182010. PMCID: PMC10172881.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Initial ABO Antibody Titer as a Variable for Estimating Number of Therapeutic Plasma Exchange prior to ABO Incompatible Kidney Transplantation
- Outcome of ABO-incompatible kidney transplantation depending on the ABO type of transfused plasma: comparative analysis between the universal AB plasma and donor type plasma
- Suggestion to Use Optimal ABO Non-Identical but Compatible RBC Components in Emergency/Massive Transfusions: Are Type O RBC the Second Best for Type AB Patients?
- ABO Gene Analysis of Discrepant ABO Blood Group in Blood Donors
- ABO gene analysis of cis-AB and B subgroup in blood donors