Ann Lab Med.
2024 Jul;44(4):343-353. 10.3343/alm.2023.0337.
TSHR Variant Screening and Phenotype Analysis in 367 Chinese Patients With Congenital Hypothyroidism
- Affiliations
-
- 1The Core Laboratory in Medical Center of Clinical Research, Department of Molecular Diagnostics & Endocrinology, Shanghai Ninth People’s Hospital, State Key Laboratory of Medical Genomics, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Department of Endocrine Metabolism, Minhang Hospital, Fudan University, Shanghai, China
- KMID: 2557933
- DOI: http://doi.org/10.3343/alm.2023.0337
Abstract
- Background
Genetic defects in the human thyroid-stimulating hormone (TSH) receptor (TSHR) gene can cause congenital hypothyroidism (CH). However, the biological functions and comprehensive genotype–phenotype relationships for most TSHR variants associated with CH remain unexplored. We aimed to identify TSHR variants in Chinese patients with CH, analyze the functions of the variants, and explore the relationships between TSHR genotypes and clinical phenotypes.
Methods
In total, 367 patients with CH were recruited for TSHR variant screening using whole-exome sequencing. The effects of the variants were evaluated by in-silico programs such as SIFT and polyphen2. Furthermore, these variants were transfected into 293T cells to detect their Gs/cyclic AMP and Gq/11 signaling activity.
Results
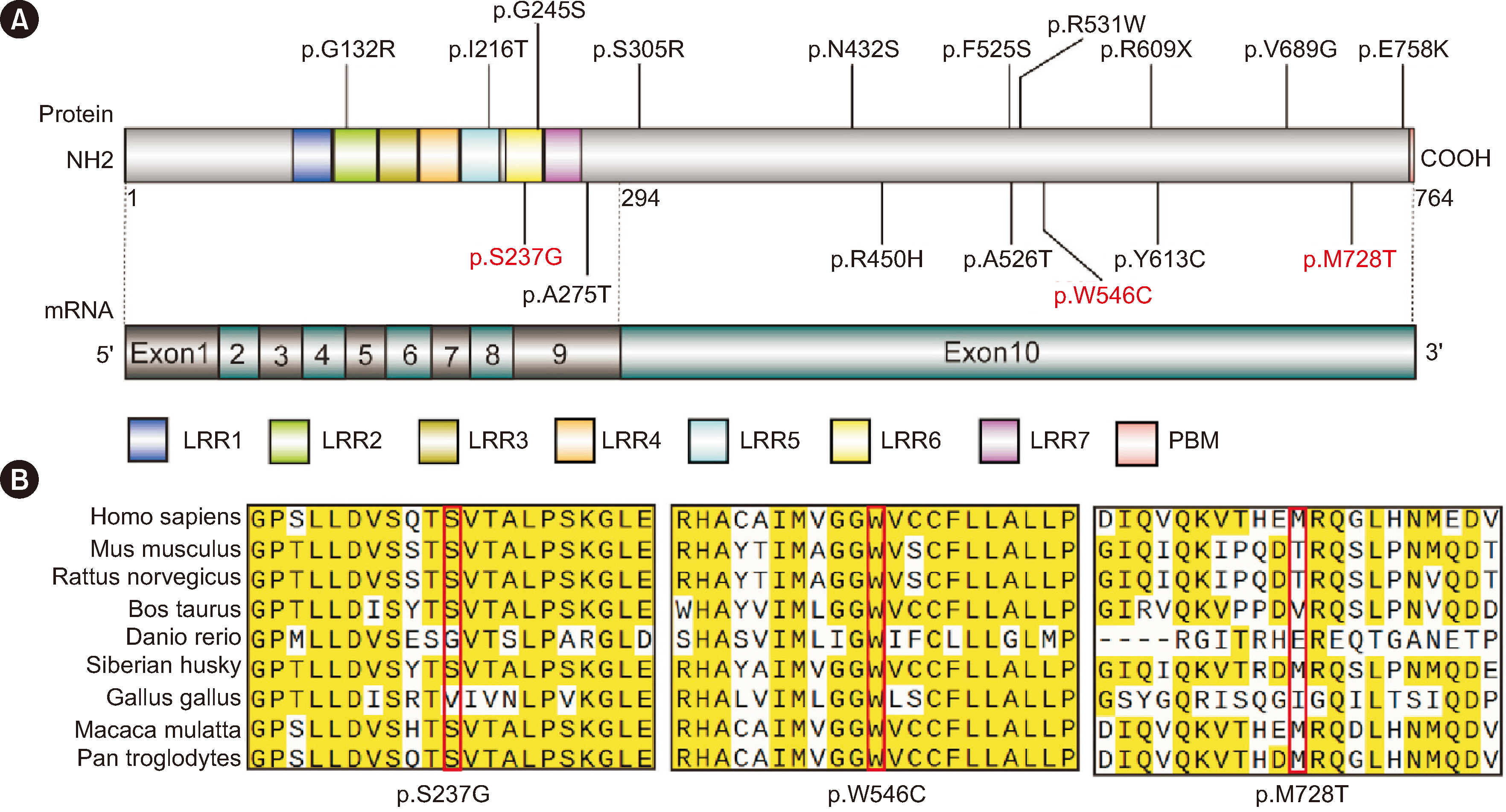
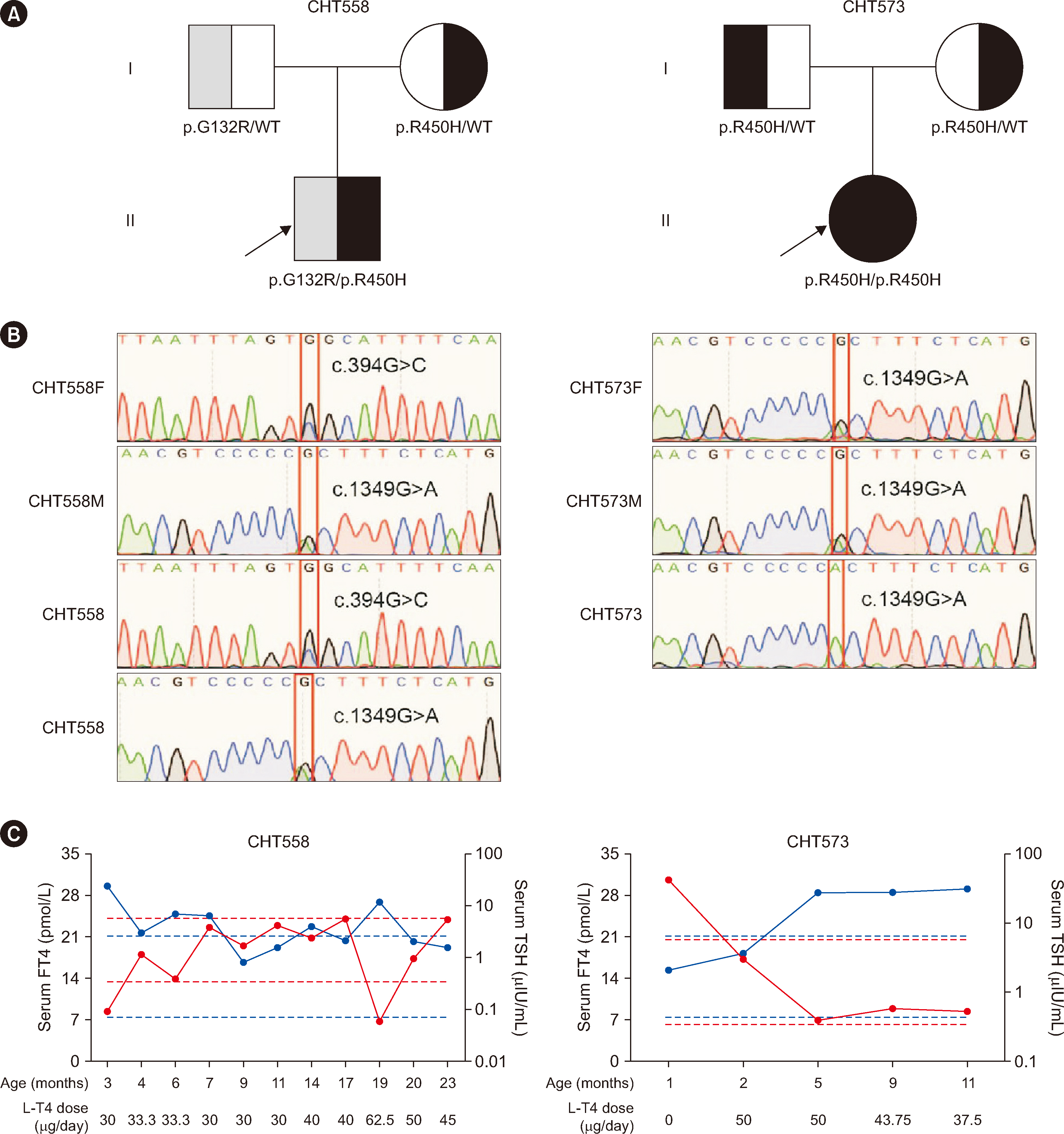
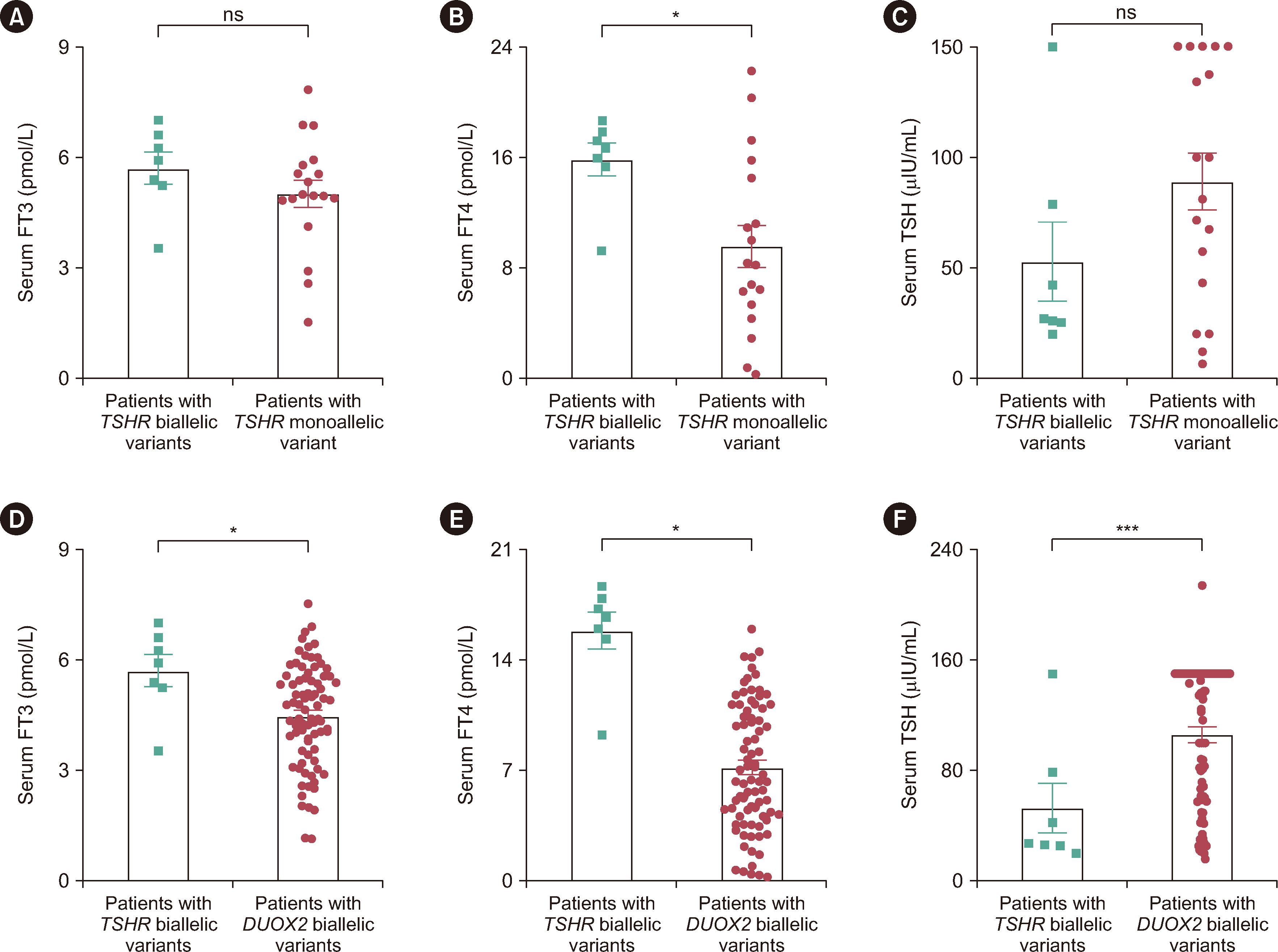
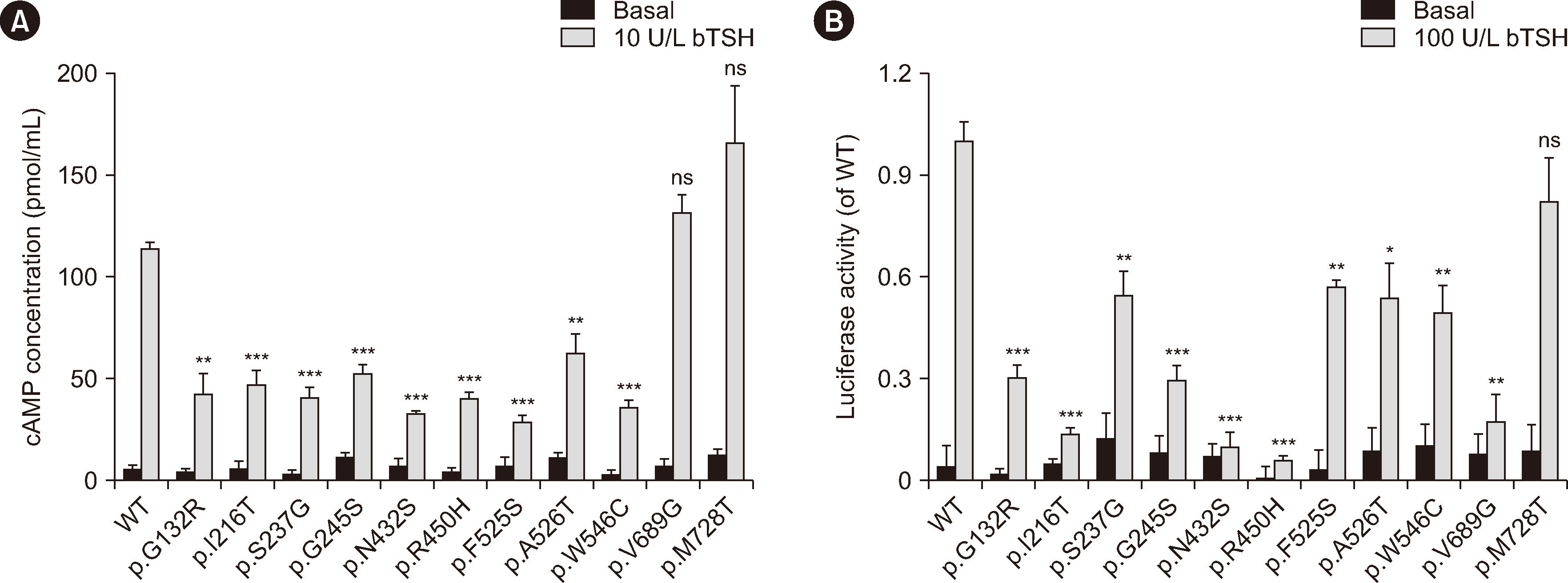
Among the 367 patients with CH, 17 TSHR variants, including three novel variants, were identified in 45 patients, and 18 patients carried biallelic TSHR variants. In vitro experiments showed that 10 variants were associated with Gs/cyclic AMP and Gq/11 signaling pathway impairment to varying degrees. Patients with TSHR biallelic variants had lower serum TSH levels and higher free triiodothyronine and thyroxine levels at diagnosis than those with DUOX2 biallelic variants.
Conclusions
We found a high frequency of TSHR variants in Chinese patients with CH (12.3%), and 4.9% of cases were caused by TSHR biallelic variants. Ten variants were identified as loss-of-function variants. The data suggest that the clinical phenotype of CH patients caused by TSHR biallelic variants is relatively mild. Our study expands the TSHR variant spectrum and provides further evidence for the elucidation of the genetic etiology of CH.
Keyword
Figure
Reference
-
References
1. van Trotsenburg P, Stoupa A, Léger J, Rohrer T, Peters C, Fugazzola L, et al. 2021; Congenital hypothyroidism: a 2020-2021 consensus guidelines update-an ENDO-European Reference Network Initiative endorsed by the European Society for Pediatric Endocrinology and the European Society for Endocrinology. Thyroid. 31:387–419. DOI: 10.1089/thy.2020.0333. PMID: 33272083. PMCID: PMC8001676.
Article2. Kostopoulou E, Miliordos K, Spiliotis B. 2021; Genetics of primary congenital hypothyroidism-a review. Hormones (Athens). 20:225–36. DOI: 10.1007/s42000-020-00267-x. PMID: 33400193.
Article3. Stoupa A, Kariyawasam D, Muzza M, de Filippis T, Fugazzola L, Polak M, et al. 2021; New genetics in congenital hypothyroidism. Endocrine. 71:696–705. DOI: 10.1007/s12020-021-02646-9. PMID: 33650047.
Article4. Winkler F, Kleinau G, Tarnow P, Rediger A, Grohmann L, Gaetjens I, et al. 2010; A new phenotype of nongoitrous and nonautoimmune hyperthyroidism caused by a heterozygous thyrotropin receptor mutation in transmembrane helix 6. J Clin Endocrinol Metab. 95:3605–10. DOI: 10.1210/jc.2010-0112. PMID: 20501679.
Article5. Narumi S, Nagasaki K, Ishii T, Muroya K, Asakura Y, Adachi M, et al. 2011; Nonclassic TSH resistance: TSHR mutation carriers with discrepantly high thyroidal iodine uptake. J Clin Endocrinol Metab. 96:E1340–5. DOI: 10.1210/jc.2011-0070. PMID: 21677043.6. Kero J, Ahmed K, Wettschureck N, Tunaru S, Wintermantel T, Greiner E, et al. 2007; Thyrocyte-specific Gq/G11 deficiency impairs thyroid function and prevents goiter development. J Clin Invest. 117:2399–407. DOI: 10.1172/JCI30380. PMID: 17694176. PMCID: PMC1937498.
Article7. Persani L, Calebiro D, Cordella D, Weber G, Gelmini G, Libri D, et al. 2010; Genetics and phenomics of hypothyroidism due to TSH resistance. Mol Cell Endocrinol. 322:72–82. DOI: 10.1016/j.mce.2010.01.008. PMID: 20083154.
Article8. Alberti L, Proverbio MC, Costagliola S, Romoli R, Boldrighini B, Vigone MC, et al. 2002; Germline mutations of TSH receptor gene as cause of nonautoimmune subclinical hypothyroidism. J Clin Endocrinol Metab. 87:2549–55. DOI: 10.1210/jcem.87.6.8536. PMID: 12050212.
Article9. Schoenmakers N, Chatterjee VK. 2015; Thyroid gland: TSHR mutations and subclinical congenital hypothyroidism. Nat Rev Endocrinol. 11:258–9. DOI: 10.1038/nrendo.2015.27. PMID: 25707783.
Article10. Grasberger H, Refetoff S. 2017; Resistance to thyrotropin. Best Pract Res Clin Endocrinol Metab. 31:183–94. DOI: 10.1016/j.beem.2017.03.004. PMID: 28648507. PMCID: PMC5569899.
Article11. Cassio A, Nicoletti A, Rizzello A, Zazzetta E, Bal M, Baldazzi L. 2013; Current loss-of-function mutations in the thyrotropin receptor gene: when to investigate, clinical effects, and treatment. J Clin Res Pediatr Endocrinol. 5 Suppl 1:29–39.
Article12. Sunthornthepvarakul T, Gottschalk ME, Hayashi Y, Refetoff S. 1995; Brief report: resistance to thyrotropin caused by mutations in the thyrotropin-receptor gene. N Engl J Med. 332:155–60. DOI: 10.1056/NEJM199501193320305. PMID: 7528344.
Article13. Fang Y, Sun F, Zhang RJ, Zhang CR, Yan CY, Zhou Z, et al. 2019; Mutation screening of the TSHR gene in 220 Chinese patients with congenital hypothyroidism. Clin Chim Acta. 497:147–52. DOI: 10.1016/j.cca.2019.07.031. PMID: 31356790.14. Sun F, Zhang JX, Yang CY, Gao GQ, Zhu WB, Han B, et al. 2018; The genetic characteristics of congenital hypothyroidism in China by comprehensive screening of 21 candidate genes. Eur J Endocrinol. 178:623–33. DOI: 10.1530/EJE-17-1017. PMID: 29650690. PMCID: PMC5958289.
Article15. Yang RM, Zhan M, Zhou QY, Ye XP, Wu FY, Dong M, et al. 2021; Upregulation of GBP1 in thyroid primordium is required for developmental thyroid morphogenesis. Genet Med. 23:1944–51. DOI: 10.1038/s41436-021-01237-3. PMID: 34194003. PMCID: PMC8486662.
Article16. Léger J, Olivieri A, Donaldson M, Torresani T, Krude H, van Vliet G, et al. 2014; European Society for Paediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. J Clin Endocrinol Metab. 99:363–84. DOI: 10.1210/jc.2013-1891. PMID: 24446653. PMCID: PMC4207909.
Article17. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. 2015; Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 17:405–24. DOI: 10.1038/gim.2015.30. PMID: 25741868. PMCID: PMC4544753.
Article18. Sun F, Zhang RJ, Cheng F, Fang Y, Yang RM, Ye XP, et al. 2021; Correlation of DUOX2 residual enzymatic activity with phenotype in congenital hypothyroidism caused by biallelic DUOX2 defects. Clin Genet. 100:713–21. DOI: 10.1111/cge.14065. PMID: 34564849.19. Fu C, Wang J, Luo S, Yang Q, Li Q, Zheng H, et al. 2016; Next-generation sequencing analysis of TSHR in 384 Chinese subclinical congenital hypothyroidism (CH) and CH patients. Clin Chim Acta. 462:127–32. DOI: 10.1016/j.cca.2016.09.007. PMID: 27637299.20. Wang H, Kong X, Pei Y, Cui X, Zhu Y, He Z, et al. 2020; Mutation spectrum analysis of 29 causative genes in 43 Chinese patients with congenital hypothyroidism. Mol Med Rep. 22:297–309. DOI: 10.3892/mmr.2020.11078. PMID: 32319661. PMCID: PMC7248516.
Article21. Fan X, Fu C, Shen Y, Li C, Luo S, Li Q, et al. 2017; Next-generation sequencing analysis of twelve known causative genes in congenital hypothyroidism. Clin Chim Acta. 468:76–80. DOI: 10.1016/j.cca.2017.02.009. PMID: 28215547.
Article22. Shin JH, Kim HY, Kim YM, Lee H, Bae MH, Park KH, et al. 2021; Genetic evaluation of congenital hypothyroidism with gland in situ using targeted exome sequencing. Ann Clin Lab Sci. 51:73–81. DOI: 10.5005/jp/books/13094_9.23. Nicoletti A, Bal M, De Marco G, Baldazzi L, Agretti P, Menabò S, et al. 2009; Thyrotropin-stimulating hormone receptor gene analysis in pediatric patients with non-autoimmune subclinical hypothyroidism. J Clin Endocrinol Metab. 94:4187–94. DOI: 10.1210/jc.2009-0618. PMID: 19820021.
Article24. Narumi S, Muroya K, Abe Y, Yasui M, Asakura Y, Adachi M, et al. 2009; TSHR mutations as a cause of congenital hypothyroidism in Japan: a population-based genetic epidemiology study. J Clin Endocrinol Metab. 94:1317–23. DOI: 10.1210/jc.2008-1767. PMID: 19158199.25. Ma SG, Fang PH, Hong B, Yu WN. 2010; The R450H mutation and D727E polymorphism of the thyrotropin receptor gene in a Chinese child with congenital hypothyroidism. J Pediatr Endocrinol Metab. 23:1339–44. DOI: 10.1515/jpem.2010.209.
Article26. Park SM, Clifton-Bligh RJ, Betts P, Chatterjee VK. 2004; Congenital hypothyroidism and apparent athyreosis with compound heterozygosity or compensated hypothyroidism with probable hemizygosity for inactivating mutations of the TSH receptor. Clin Endocrinol (Oxf). 60:220–7. DOI: 10.1111/j.1365-2265.2004.01967.x. PMID: 14725684.
Article27. Mueller S, Szkudlinski MW, Schaarschmidt J, Günther R, Paschke R, Jaeschke H. 2011; Identification of novel TSH interaction sites by systematic binding analysis of the TSHR hinge region. Endocrinology. 152:3268–78. DOI: 10.1210/en.2011-0153. PMID: 21628383.28. Chazenbalk GD, Nagayama Y, Russo D, Wadsworth HL, Rapoport B. 1990; Functional analysis of the cytoplasmic domains of the human thyrotropin receptor by site-directed mutagenesis. J Biol Chem. 265:20970–5. DOI: 10.1016/S0021-9258(17)45312-9. PMID: 2250002.
Article29. Sugisawa C, Abe K, Sunaga Y, Taniyama M, Hasegawa T, Narumi S. 2018; Identification of compound heterozygous TSHR mutations (R109Q and R450H) in a patient with nonclassic TSH resistance and functional characterization of the mutant receptors. Clin Pediatr Endocrinol. 27:123–30. DOI: 10.1297/cpe.27.123. PMID: 30083029. PMCID: PMC6073063.
Article30. Vigone MC, Fugazzola L, Zamproni I, Passoni A, Di Candia S, Chiumello G, et al. 2005; Persistent mild hypothyroidism associated with novel sequence variants of the DUOX2 gene in two siblings. Hum Mutat. 26:395. DOI: 10.1002/humu.9372. PMID: 16134168.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Cost-benefit Analysis on Neonatal Screening of Phenylketonuria and Congenital Hypothyroidism in Korea
- Genetic Variations of Congenital Hypothyroidism
- Two Cases with Prolonged TSH Elevation in Congenital Hypothyroidism
- Hypothyroidism
- Characteristics of Transient Hypothyroidism Detected by Neonatal Screening Test