Ann Clin Microbiol.
2024 Jun;27(2):49-67. 10.5145/ACM.2024.27.2.3.
Practical guide for the diagnosis of helminth ova in stools
- Affiliations
-
- 1Department of Parasitology and Tropical Medicine, Institute of Health Sciences, Gyeongsang National University College of Medicine, Jinju, Korea
- 2Department of Tropical Medicine and Parasitology, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2557878
- DOI: http://doi.org/10.5145/ACM.2024.27.2.3
Abstract
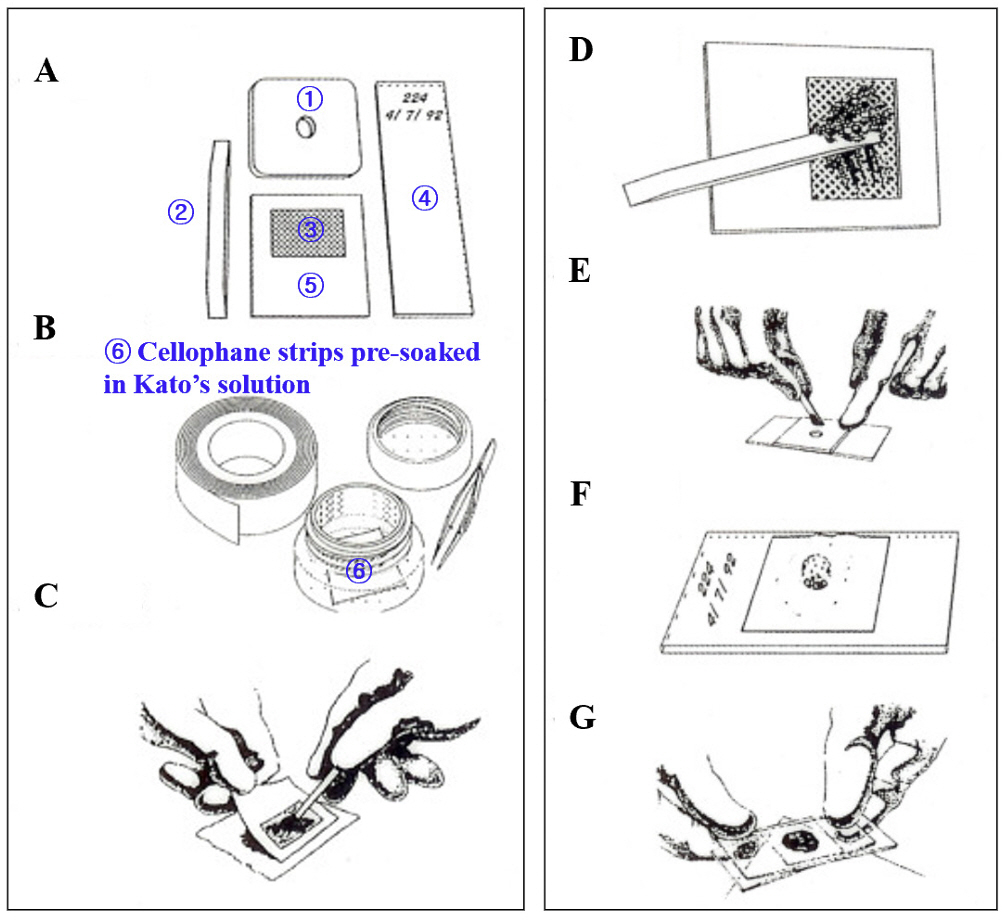
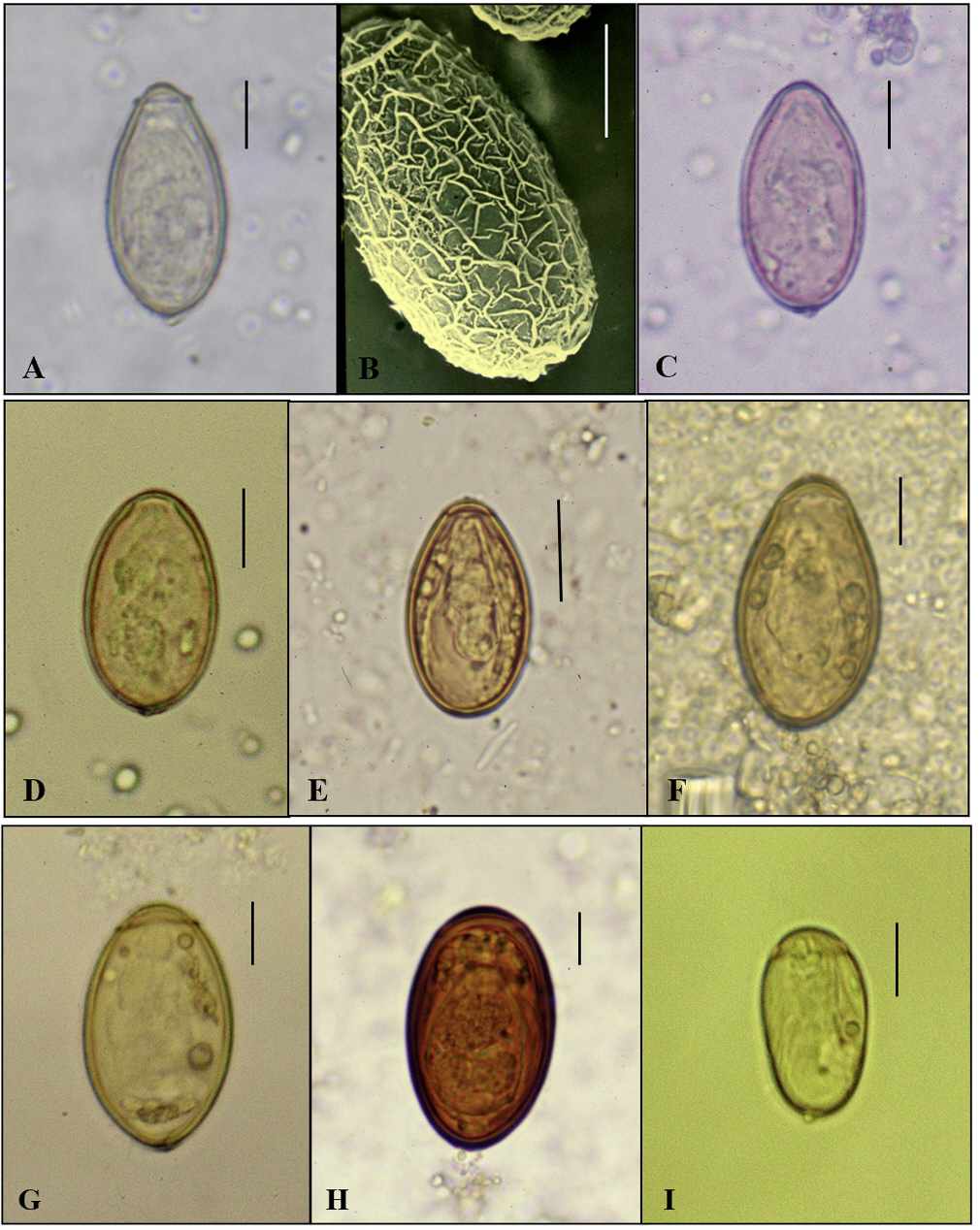
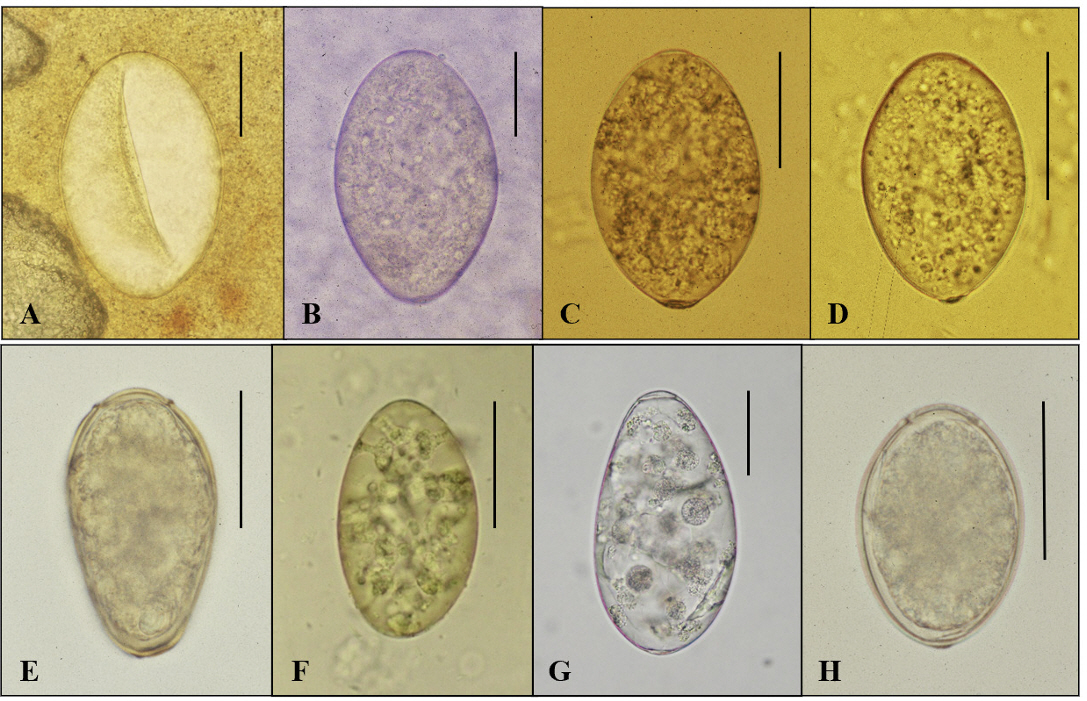
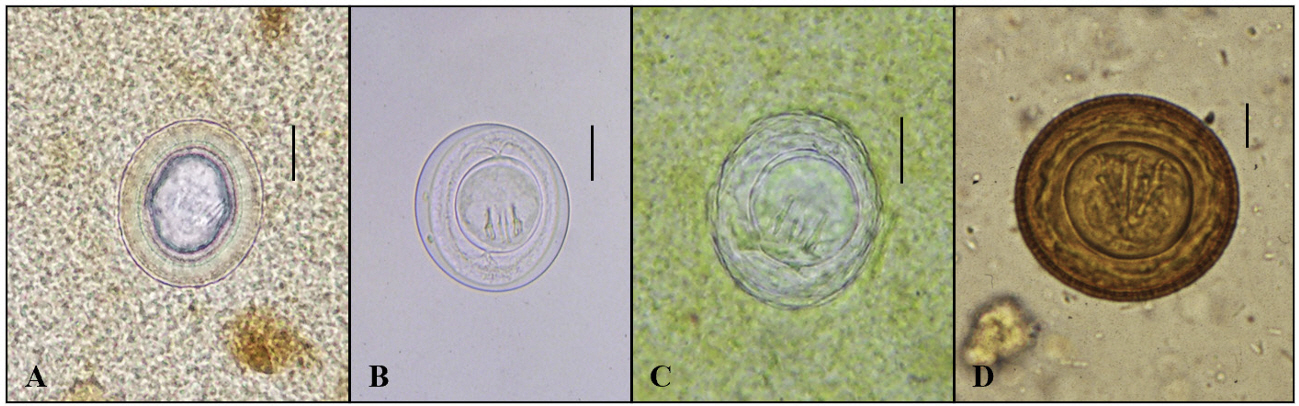
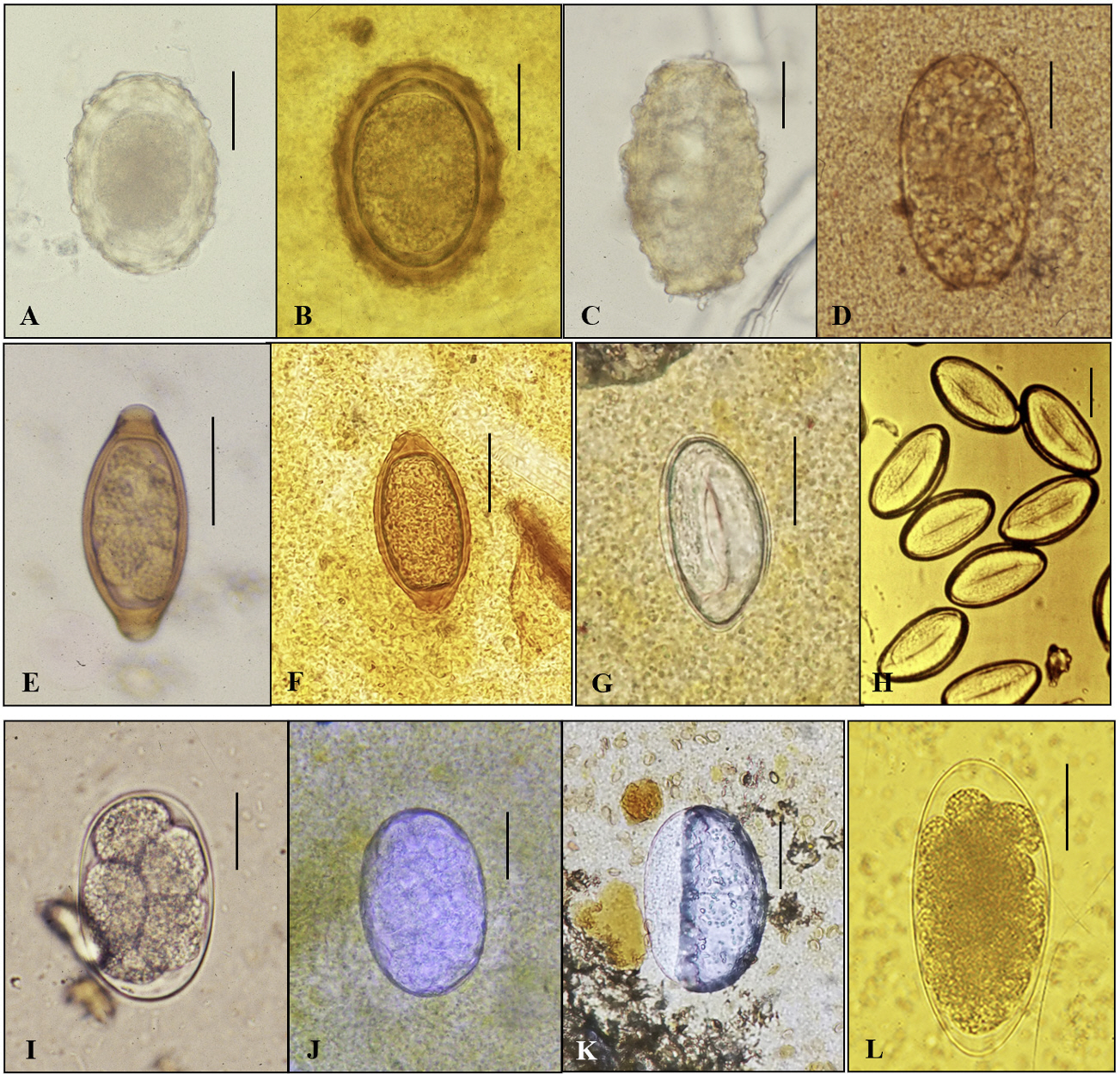
- In the age of globalization of infectious diseases, qualified personnel is needed for the diagnosis of parasitic diseases in the laboratory. This review aimed to introduce the methods for stool examination and identification of helminth eggs for the diagnosis of helminthic infections in laboratory and field surveys. The formalin-ether sedimentation technique (FEST) and the Kato-Katz egg counting technique (KKECT) are mainly described as representative stool examinations. The FEST is somewhat complicated and troublesome, but it is useful for differentiating small trematode eggs from opisthorchiid and heterophyid flukes. KKECT is useful in field surveys of large populations in areas endemic for soil-transmitted helminthiases. Helminth eggs are divided into four groups based on the presence or absence of the operculum and embryo. The eggs of Clonorchis sinensis and heterophyid flukes including Metagonimus spp. are relatively small and contain an operculum and an embryo (miracidium). Meanwhile, eggs of diphyllobothriid tapeworms, echinostomatid flukes, Paragonimus westermani, Fasciola hepatica, and Fasciolopsis buski are relatively larger, operculated, and contain germ cells and yolks instead of the embryo. The eggs of cyclophyllidian tapeworms, Taenia spp. and Hymenolepis spp., and blood flukes, Schistosoma spp., are embryonated but do not have an operculum. Nematode eggs have no operculum and embryo, but those of hookworms and pinworms sometimes have developed larvae inside. This review provides valuable insights into the methods of stool examination and helminth egg identification for the diagnosis of helminthic infections in the laboratory and field surveys.
Keyword
Figure
Reference
-
1. Seo BS. Clinical parasitology. 2nd ed. Seoul; Ilchokak, 1978: 360-70. .2. Beaver PC, Jung R, et al. eds. Clinical pathophysiology. 9th ed. Philadelphia; Lea & Febiger, 1984: 733-48. .3. Chai JY, Hong ST, et al. eds. Seo and Lee’s clinical parasitology. Seoul; Seoul National University Press, 2011: 22-7. .4. Mohd-Zain SN, Rahman R, Lewis JW. Stray animals and human defecation are sources of soil-transmitted helminth eggs in playgrounds in Peninsular Malaysia. J Helminthol 2015;89:740-7. .5. Campos MC, Beltrán M, Fuentes N, Moreno G. Helminth eggs as parasitic indicators of fecal contamination in agricultural irrigation water, biosolids, soils, and pastures. Biomedica 2018;38:42-53. .6. Chai JY, Seo M, Shin DH. Paleoparasitological research on ancient helminth eggs and larvae in the Republic of Korea. Parasites Hosts Dis 2023;61:345-87 .7. Korea Centers for Disease Control and Prevention. A national survey of the prevalence of intestinal parasitic infections in Korea in 2012. The 8th Report. Osong: Korea National Institutes of Health; 2013. .8. Seo BS, Lee SH, Cho SY, Chai JY, Hong ST, Han IS, et al. An epidemiological study on clonorchiasis and metagonimiasis in riverside areas of Korea. Korean J Parasitol 1981;19:13750. .9. Cho SH, Lee KY, Lee BC, Cho PY, Cheun HI, Hong ST, et al. Prevalence of clonorchiasis in the southern endemic areas of Korea in 2006. Korean J Parasitol 2008; 46:133-7. .10. Jeong YI, Shin HE, Lee SE, Cheun HI, Ju JW, Kim JY, et al. Prevalence of Clonorchis sinensis infection among residents of five major rivers in the Republic of Korea. Korean J Parasitol 2016;54:215-9. .11. Yoo WG, Sohn WM, Na BK. Current status of Clonorchis sinensis and clonorchiasis in Korea: epidemiological perspectives integrating data from humans and intermediate hosts. Parasitology 2022;149:1296-305. .12. Lee MR, Shin HE, Back SO, Lee YJ, Lee HI, Ju JW. Status of helminthic infections in residents of river basins in the Republic of Korea over 10 Years (2011-2020). Korean J Parasitol 2022;60:187-93. .13. Korean Association for Health Promotion (KAHP). KAHP annual report. Seoul: Korean Association for Health Promotion; 2021:49, 2022:34, 2023:38. .14. Ju JW, Lee MR, Shin HE, Kim YJ, Lee SE, Kim HJ, et al. Current status of the elimination project for intestinal parasitic diseases in areas endemic for food-borne trematodiasis. Week Health Dis 2017;11:426-32. .15. Chai JY, Sohn WM, Hong SJ, Jung BK, Hong SJ, Cho S, et al. Effect of mass drug administration with a single dose of Albendazole on Ascaris lumbricoides and Trichuris trichiura infections among schoolchildren in the Yangon Region, Myanmar. Korean J Parasitol 2020;58:195-200. .16. Yong TS, Chai JY, Sohn WM, Eom KS, Jeoung HG, Hoang EH, et al. Prevalence of intestinal helminths among Cambodian inhabitants (2006-2011). Korean J Parasitol 2014;52:661-6. .17. Eom KS, Yong TS, Sohn WM, Chai JY, Min DY, Rim HJ, et al. Prevalence of helminthic infections among inhabitants of Lao PDR. Korean J Parasitol 2014;52:51-6. .18. Rim HJ, Chai JY, Min DY, Cho SY, Eom KS, Hong SJ, et al. Prevalence of intestinal parasite infection on a national scale among primary school children in Laos. Parasitol Res 2003;91:267-72. .19. Kim JY, Sim SB, Cung EJ, Rim HJ, Chai JY, Min DY, et al. Effectiveness of mass drug administration for neglected tropical diseases in schoolchildren in Zanzibar, Tanzania. Korean J Parasitol 2020;58:109-19. .20. Garcia LS. Clinical microbiology procedure handbook. American Society for Microbiology Press; 2010: 2265. .21. Garcia LS, Arrowood M, Kokoskin E, Paltridge GP, Pillai DR, Procop GW, et al. Practical guidance for clinical microbiology laboratories: diagnosis of parasites in the gastrointestinal tract. Clin Microbiol Rev 2017;15;31:e00025-17. .22. Palmieri JR, Elswaifi SF, Fried KK. Emerging need for parasitology education: training to identify and diagnose parasitic infections. Am J Trop Med Hyg 2011; 84:845–6. .23. McCarthy JS, Lustigman S, Yang GJ, Barakat RM, García HH, Sripa B, et al. Research agenda for helminth diseases in humans: diagnostics for control and elimination programs. PLoS Negl Trop Dis 2012;6:e1601. .24. Boonyong S, Hunnangkul S, Vijit S, Wattano S, Tantayapirak P, Loymek S, et al. Highthroughput detection of parasites and ova in stool using a fully automatic digital feces analyzer, Orienter model fa280. Parasites Vectors 2024;17:13. .25. Lefaure B, Kebbabi C, Monnin N, Machouart M, Debourgogne A. Evaluation of a flotation adapted Parasep® for stool ova and parasite examination. J Parasitol 2019;105:480-3. .26. Sarirah M, Wijayanti MA, Murhandarwati EEH. Comparison of mini-flotac and Kato-Katz methods for detecting soil-transmitted helminth eggs in 10% formalin-preserved stools stored for > 12 months. Trop Biomed 2019;36:677–86. .27. Khanna V, Sagar S Khanna R, Chawla K. A comparative study of formalin-ethyl acetate sedimentation technique and Mini Parasep® solvent-free method in the rapid diagnosis of intestinal parasites. Trop Parasitol 2018;8:29-32. .28. World Health Organization. Bench aids for the diagnosis of intestinal parasites. 2nd ed. Geneva: WHO; 2019. .29. Ryoo S, Jung BK, Hong S, Shin H, Song H, Kim HS, et al. Standard- and large-sized eggs of Trichuris trichiura in the feces of schoolchildren in the Yangon region, Myanmar: morphological and molecular analyses. Parasites Hosts Dis 2023;61:317-24. .30. Soares FA, Benitez AN, dos Santos BM, Loiola SHN, Rosa SL, Nagata WB, et al. Historical review of parasite recovery techniques for detection in human stools. Rev Soc Bras Med Trop 2020;53:e20190535. .31. Inácio SV, Ferreira Gomes J, Xavier Falcão A, Nagase Suzuki CT, Bertequini Nagata W, Nery Loiola SH, et al. Automated diagnosis of canine gastrointestinal parasites using image analysis. Pathogens 2020;9:139. .32. Jim´enez B, Maya C, Vel´asquez G, Barrios JA, Perez M, Román A, et al. Helminth egg automatic detector (HEAD): improvements in development for digital identification and quantification of helminth eggs and their application online. Exp Parasitol 2020;217:107959. .33. Alva A, Cangalaya C, Quiliano M, Krebs C, Gilman RH, Sheen P, et al. Mathematical algorithm for the automatic recognition of intestinal parasites. PLoS One 2017;12:e0175646. .34. Jimenez B, Maya C, Velasquez G, Torner F, Arambula F, Barrios JA, et al. Identification and quantification of pathogenic helminth eggs using a digital image system. Exp Parasitol 2016;166:164-72 .35. Yang YS, Park DK, Kim HC Choi MH, Chai JY. Automatic identification of human helminth eggs in microscopic fecal specimens using digital image processing and an artificial neural network. IEEE Trans Biomed Eng 2001;48:718–30. .
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An evaluation of cellophane thick smear technique for mass stool examination
- Hatching and activation of some cestode ova - The effects of various artificial hatching-activating solutions upon the some cestode ova
- A case report of intestinal eosinophilic granuloma du to Ascaris Ova
- Truly practical, a book review for Teaching Anatomy: A Practical Guide
- Egg discharging patterns of Ascaris lumbricoides in low worm burden cases