J Korean Med Sci.
2024 Jul;39(25):e193. 10.3346/jkms.2024.39.e193.
Establishing a Framework for Evaluating the Effectiveness of Vaccines Targeting National Vaccination Programs
- Affiliations
-
- 1Allergy and Immunology Institute, Korea University, Seoul, Korea
- 2Department of Pediatrics, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea
- 3Division of Immunization Planning, Korea Disease Control and Prevention Agency, Cheongju, Korea
- 4Department of Pediatrics, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- KMID: 2557770
- DOI: http://doi.org/10.3346/jkms.2024.39.e193
Abstract
- Background
The increasing number of vaccines and the complexity of immunization programs, along with continuous changes in the epidemiology of infectious diseases, necessitate a systematic approach to vaccine effectiveness (VE) evaluation. This study presents a preliminary survey to establish a VE evaluation framework in Korea, focusing on the National Immunization Program.
Methods
Experts’ opinions were collected through a two-round online survey targeting key stakeholders. The first round consisted of two multiple-choice questions and two openended questions. The second round was a quantitative survey with 17 questionnaires based on five domains derived by analyzing the results of the first-round survey.
Results
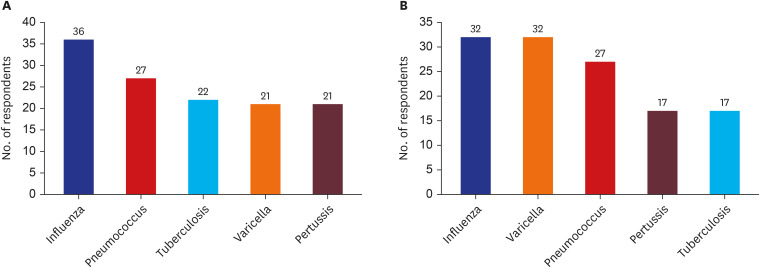
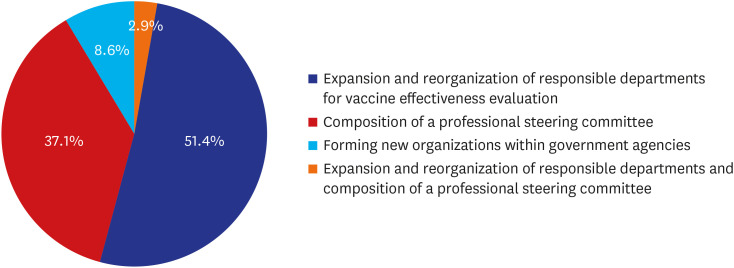
The results emphasize the necessity and urgency of a government-led VE evaluation system and the establishment of a multidisciplinary evaluation organization. Key considerations include personnel, budget, data integration, legal standards, and surveillance system enhancements.
Conclusion
These findings provide valuable insights for policymakers, emphasizing the need for collaboration, financial support, and robust data management in developing evidence-based vaccination policies.
Keyword
Figure
Reference
-
1. Crowcroft NS, Klein NP. A framework for research on vaccine effectiveness. Vaccine. 2018; 36(48):7286–7293. PMID: 30431002.2. Patel C, Rendell N, Sargent GM, Ali A, Morgan C, Fields R, et al. Measuring national immunization system performance: a systematic assessment of available resources. Glob Health Sci Pract. 2023; 11(3):e220055. PMID: 37348935.3. Choi JH, Moon J, Kim S, Bae H, Lee J, Choe YJ. Expert consensus on COVID-19 vaccination in Korean adolescents: a modified Delphi survey. J Korean Med Sci. 2022; 37(9):e69. PMID: 35257524.4. Kesselheim AS, Darrow JJ, Kulldorff M, Brown BL, Mitra-Majumdar M, Lee CC, et al. An overview of vaccine development, approval, and regulation, with implications for COVID-19: analysis reviews the Food and Drug Administration’s critical vaccine approval role with implications for COVID-19 vaccines. Health Aff (Millwood). 2021; 40(1):25–32. PMID: 33211535.5. Centers for Disease Control and Prevention. NCIRD organizational structure. Updated 2024. Accessed May 6, 2024. https://www.cdc.gov/ncird/organizational-structure.html .6. Centers for Disease Control and Prevention. ACIP Work Groups. Updated 2023. Accessed May 6, 2024. https://www.cdc.gov/vaccines/acip/workgroups.html .7. United Kingdom Health Security Agency. Updated 2023. Accessed May 6, 2024. https://www.gov.uk/government/collections/immunisation .8. Public Health Agency Data. Surveillance data. Accessed May 6, 2024. https://www.publichealth.hscni.net/directorate-public-health/health-protection/surveillance-data .9. Tessier E, Edelstein M, Tsang C, Kirsebom F, Gower C, Campbell CN, et al. Monitoring the COVID-19 immunisation programme through a National Immunisation Management System - England’s experience. Int J Med Inform. 2023; 170:104974. PMID: 36577202.10. Fukuda H, Maeda M, Murata F. Development of a COVID-19 vaccine effectiveness and safety assessment system in Japan: the VENUS study. Vaccine. 2023; 41(23):3556–3563. PMID: 37037707.11. Marzouk M, Omar M, Sirison K, Ananthakrishnan A, Durrance-Bagale A, Pheerapanyawaranun C, et al. Monitoring and evaluation of national vaccination implementation: a scoping review of how frameworks and indicators are used in the public health literature. Vaccines (Basel). 2022; 10(4):567. PMID: 35455316.12. Edelstein M, Lee LM, Herten-Crabb A, Heymann DL, Harper DR. Strengthening global public health surveillance through data and benefit sharing. Emerg Infect Dis. 2018; 24(7):1324–1330.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Global varicella vaccination programs
- The Role of Korean Medical Community in the Era of Opened Vaccination
- The development of mucosal vaccines for both mucosal and systemic immune induction and the roles played by adjuvants
- Suggested guidelines for vaccination of pigs in Korea
- Knowledge Level of Human Papillomavirus, Cervical Cancer and Vaccination Status among Mothers with Daughters in High School