Cancer Res Treat.
2024 Jul;56(3):856-870. 10.4143/crt.2023.1150.
Invasiveness of Upper Tract Urothelial Carcinoma: Clinical Significance and Integrative Diagnostic Strategy
- Affiliations
-
- 1Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Medical Science, Biomedical Science, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Radiology and Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 4Department of Urology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2557672
- DOI: http://doi.org/10.4143/crt.2023.1150
Abstract
- Purpose
In this study, we aimed to determine the clinicopathologic, radiologic, and molecular significance of the tumor invasiveness to further stratify the patients with high-grade (HG) upper tract urothelial carcinoma (UTUC) who can be treated less aggressively.
Materials and Methods
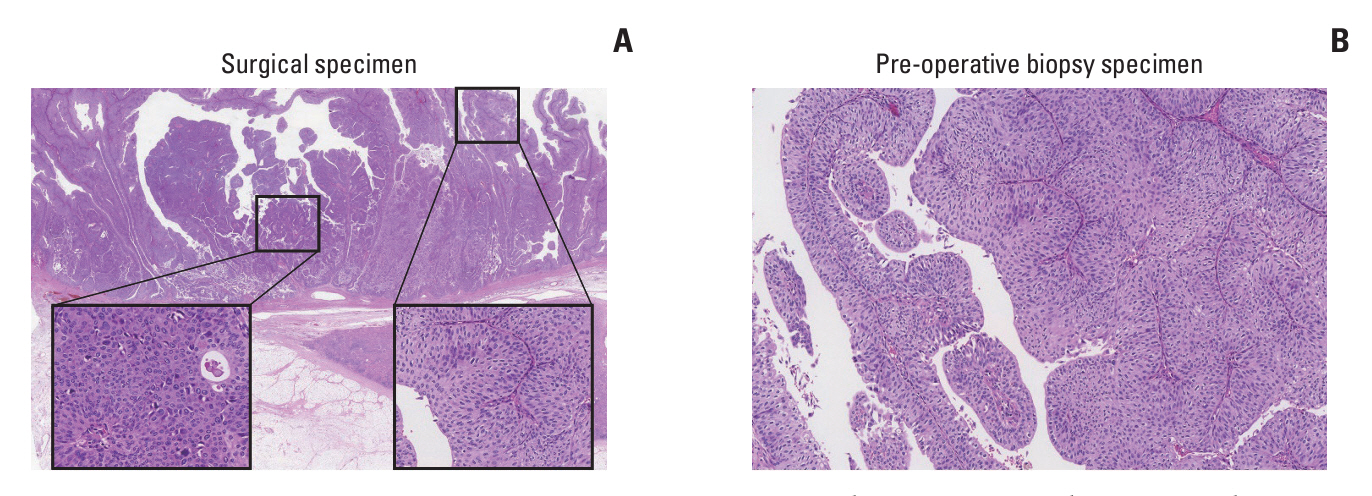
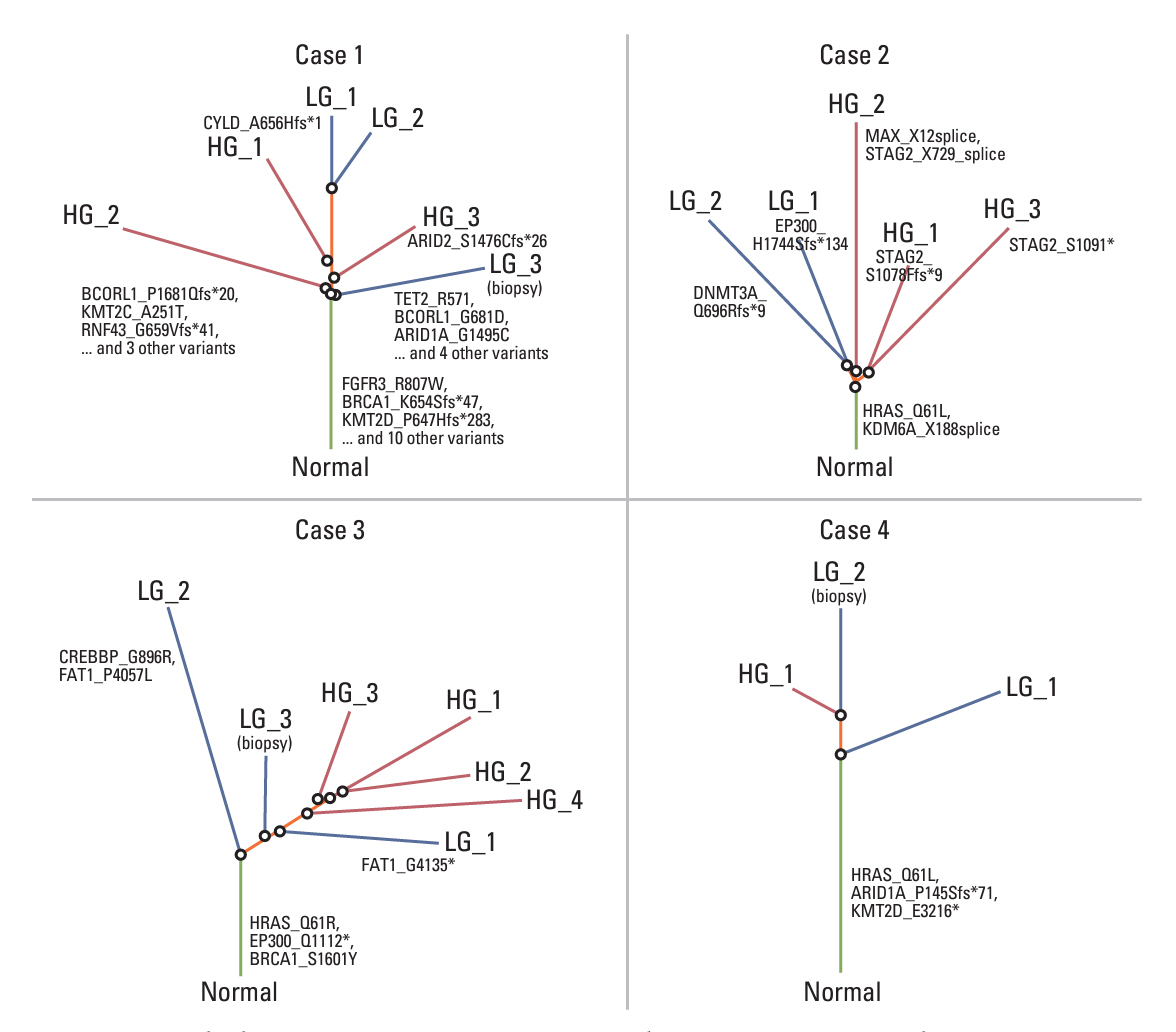
Clinicopathologic and radiologic characteristics of 166 surgically resected HG UTUC (48 noninvasive, and 118 invasive) cases were evaluated. Six noninvasive UTUC cases with intratumoral tumor grade heterogeneity were selected for whole-exome sequencing (WES) to understand the underlying molecular pathophysiology. Barcode-tagging sequencing was done for validation of the target genes from WES data.
Results
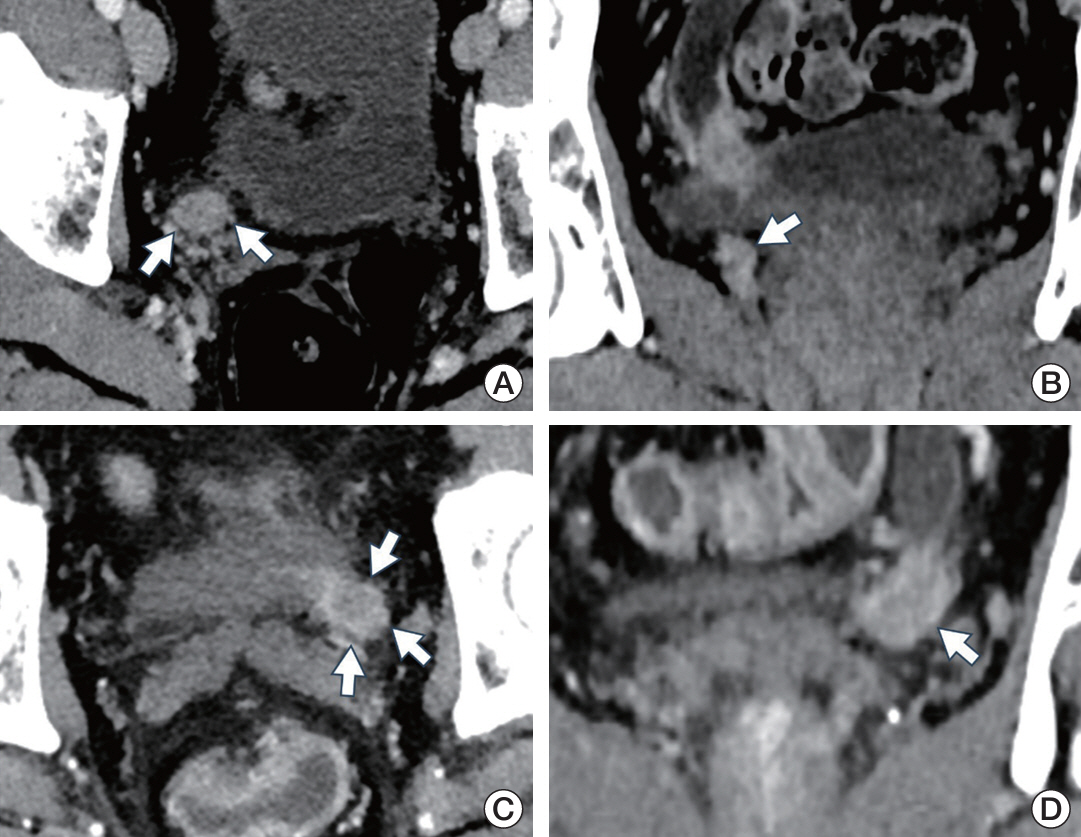
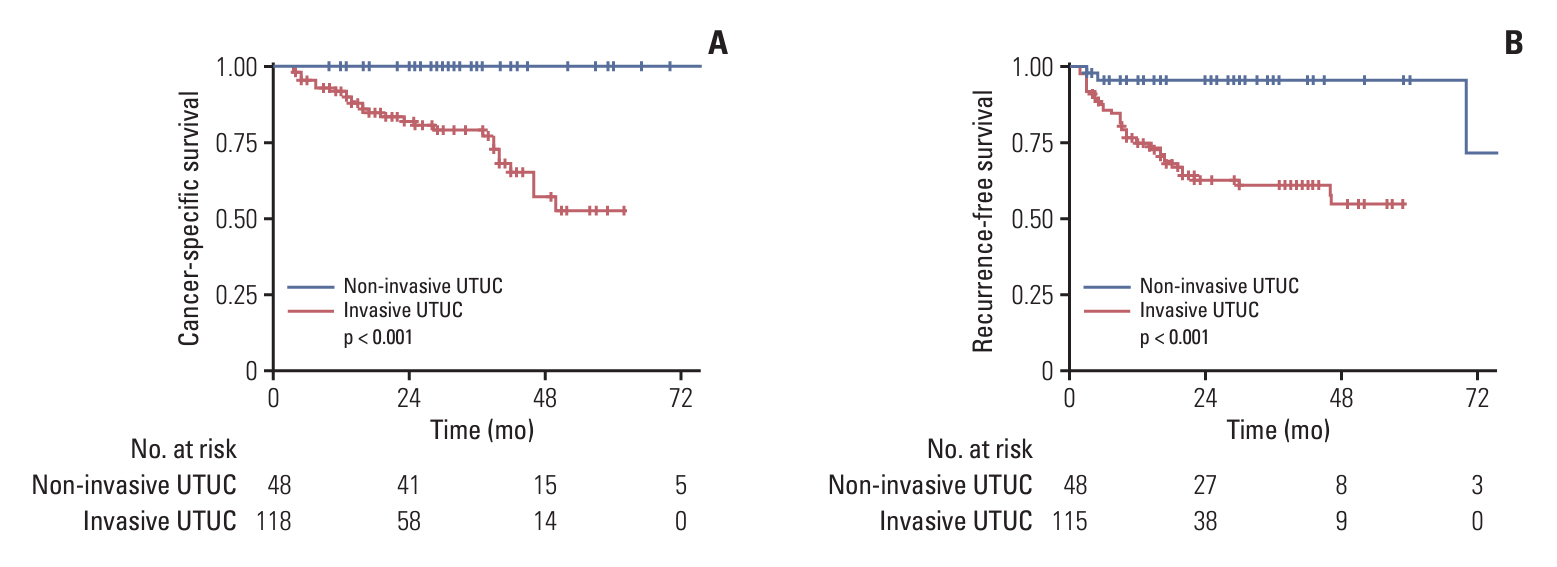
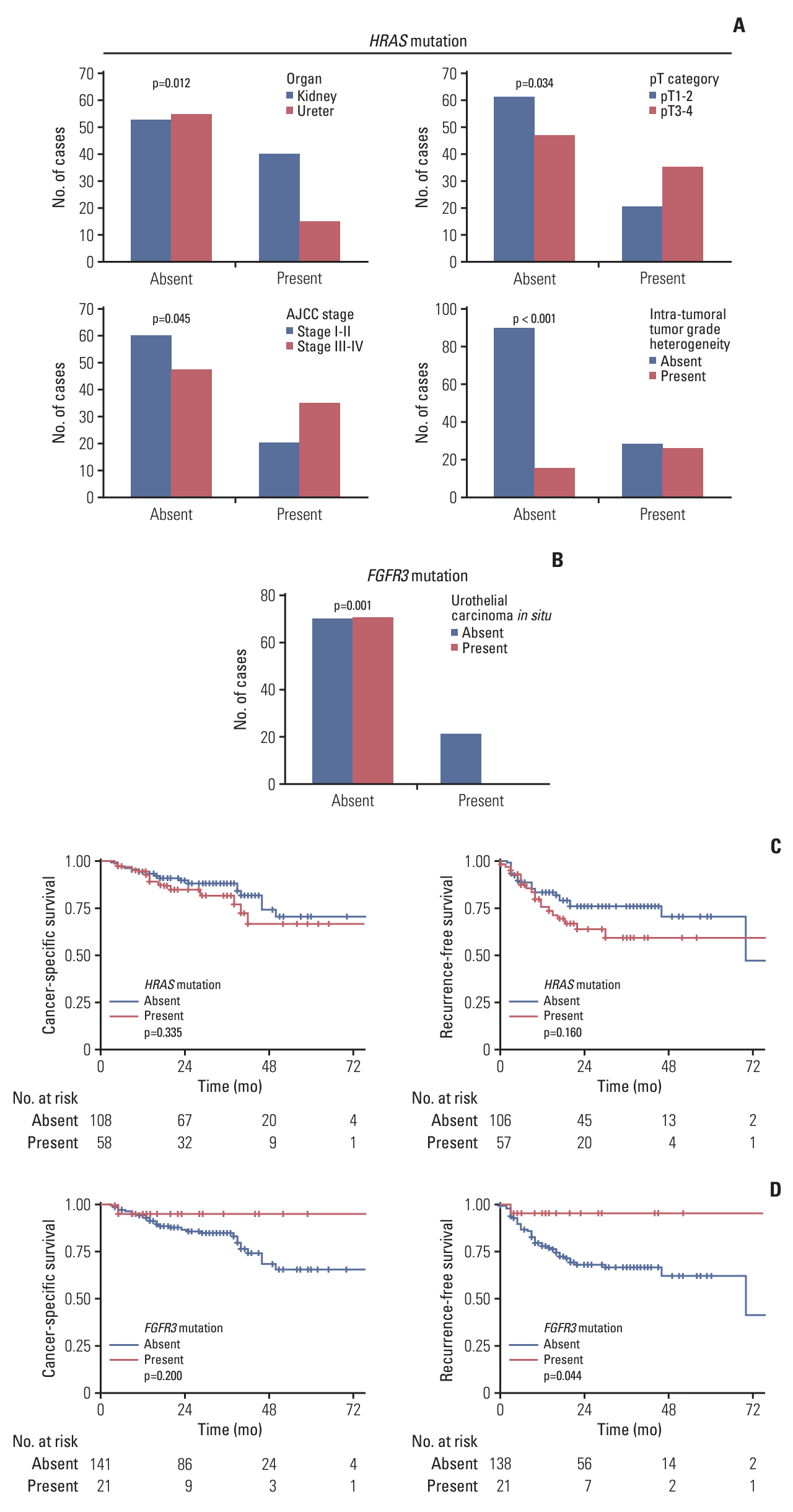
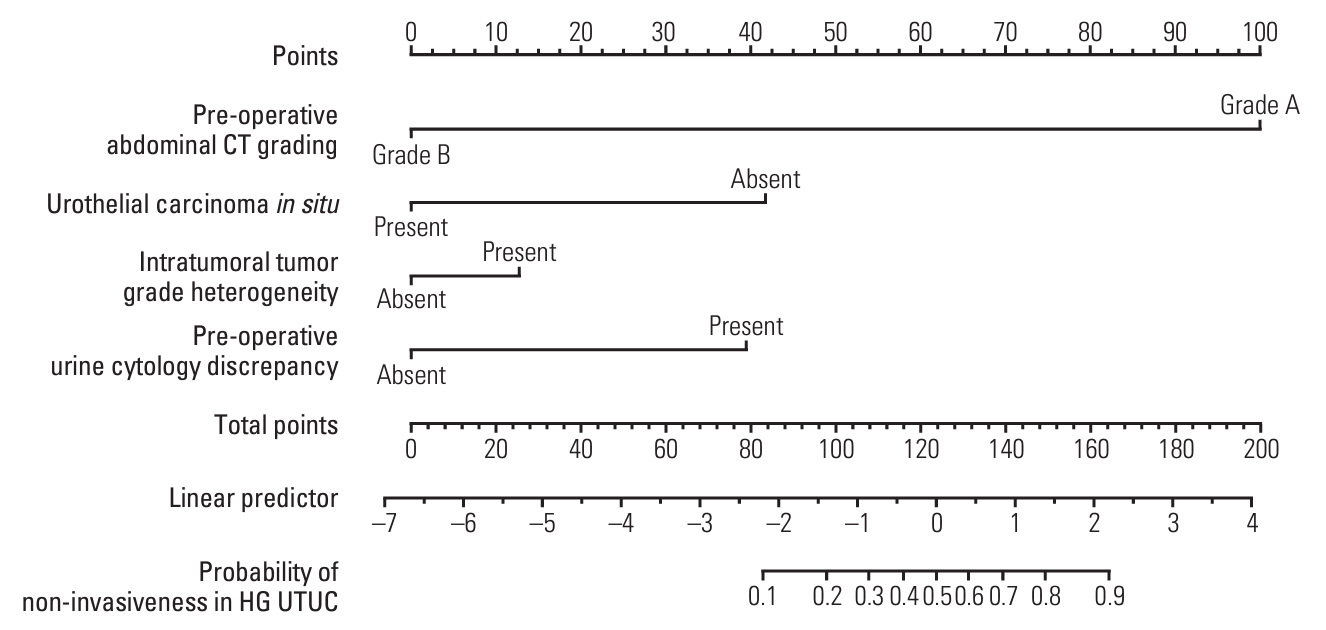
Patients with noninvasive UTUC showed no cancer-specific death with better cancer-specific survival (p < 0.001) and recurrence-free survival (p < 0.001) compared to the patients with invasive UTUC. Compared to the invasive UTUC, noninvasive UTUC was correlated to a low grade (LG) on the preoperative abdominal computed tomography (CT) grading system (p < 0.001), histologic intratumoral tumor grade heterogeneity (p=0.018), discrepancy in preoperative urine cytology diagnosis (p=0.018), and absence of urothelial carcinoma in situ (p < 0.001). WES of the heterogeneous components showed mutually shared HRAS and FGFR3 mutations shared between the HG and LG components. HRAS mutation was associated with the lower grade on preoperative abdominal CT and intratumoral tumor grade heterogeneity (p=0.045 and p < 0.001, respectively), whereas FGFR3 mutation was correlated to the absence of carcinoma in situ (p < 0.001).
Conclusion
According to our comprehensive analysis, HG noninvasive UTUC can be preoperatively suspected based on distinct preoperative radiologic, cytologic, histologic, and molecular features. Noninvasive HG UTUC shows excellent prognosis and thus should be treated less aggressively.
Keyword
Figure
Reference
-
References
1. Roupret M, Babjuk M, Comperat E, Zigeuner R, Sylvester R, Burger M, et al. European guidelines on upper tract urothelial carcinomas: 2013 update. Eur Urol. 2013; 63:1059–71.2. Leow JJ, Liu Z, Tan TW, Lee YM, Yeo EK, Chong YL. Optimal management of upper tract urothelial carcinoma: current perspectives. Onco Targets Ther. 2020; 13:1–15.3. Seisen T, Peyronnet B, Dominguez-Escrig JL, Bruins HM, Yuan CY, Babjuk M, et al. Oncologic outcomes of kidney-sparing surgery versus radical nephroureterectomy for upper tract urothelial carcinoma: a systematic review by the EAU Non-muscle Invasive Bladder Cancer Guidelines Panel. Eur Urol. 2016; 70:1052–68.4. Chen L, He H, Zarka MA, Zhou M, Magi-Galluzzi C. Upper tract urinary cytology to detect upper tract urothelial carcinoma: Using the Johns Hopkins Hospital template and evaluation of its feasibility. Cytojournal. 2015; 12:17.5. Honda Y, Nakamura Y, Teishima J, Goto K, Higaki T, Narita K, et al. Clinical staging of upper urinary tract urothelial carcinoma for T staging: Review and pictorial essay. Int J Urol. 2019; 26:1024–32.6. Honda Y, Goto K, Sentani K, Yasui W, Ikeda K, Matsubara A, et al. T categorization of urothelial carcinomas of the ureter with CT: preliminary study of new diagnostic criteria proposed for differentiating T2 or lower from T3 or higher. AJR Am J Roentgenol. 2015; 204:792–7.7. Sfakianos JP, Cha EK, Iyer G, Scott SN, Zabor EC, Shah RH, et al. Genomic characterization of upper tract urothelial carcinoma. Eur Urol. 2015; 68:970–7.
Article8. Moss TJ, Qi Y, Xi L, Peng B, Kim TB, Ezzedine NE, et al. Comprehensive genomic characterization of upper tract urothelial carcinoma. Eur Urol. 2017; 72:641–9.
Article9. Fujii Y, Sato Y, Suzuki H, Kakiuchi N, Yoshizato T, Lenis AT, et al. Molecular classification and diagnostics of upper urinary tract urothelial carcinoma. Cancer Cell. 2021; 39:793–809.
Article10. WHO Classification of Tumours Editorial Board. WHO classification of tumours: urinary and male genital tumours. 5th ed. Lyon: International Agency for Research on Cancer;2022.11. Li X, Cui M, Gu X, Fang D, Li H, Qin S, et al. Pattern and risk factors of local recurrence after nephroureterectomy for upper tract urothelial carcinoma. World J Surg Oncol. 2020; 18:114.
Article12. Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009; 25:1754–60.13. McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010; 20:1297–303.
Article14. Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 2013; 31:213–9.
Article15. Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012; 2:401–4.
Article16. Therneau TM, Grambsch PM, Fleming TR. Martingale-based residuals for survival models. Biometrika. 1990; 77:147–60.
Article17. Harrell FE Jr. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis. Cham: Springer International Publishing;2015.18. Bakkar AA, Wallerand H, Radvanyi F, Lahaye JB, Pissard S, Lecerf L, et al. FGFR3 and TP53 gene mutations define two distinct pathways in urothelial cell carcinoma of the bladder. Cancer Res. 2003; 63:8108–12.19. Lindgren D, Sjodahl G, Lauss M, Staaf J, Chebil G, Lovgren K, et al. Integrated genomic and gene expression profiling identifies two major genomic circuits in urothelial carcinoma. PLoS One. 2012; 7:e38863.
Article20. Lopez-Knowles E, Hernandez S, Malats N, Kogevinas M, Lloreta J, Carrato A, et al. PIK3CA mutations are an early genetic alteration associated with FGFR3 mutations in superficial papillary bladder tumors. Cancer Res. 2006; 66:7401–4.
Article21. Kittler R, Shiang C, Hutchinson R, Kollipara RK, Kapur P, Franto F, et al. Grade progression in urothelial carcinoma can occur with high or low mutational homology: a first-step toward tumor-specific care in initial low-grade bladder cancer. Oncotarget. 2018; 9:9415–24.
Article22. Robinson BD, Vlachostergios PJ, Bhinder B, Liu W, Li K, Moss TJ, et al. Upper tract urothelial carcinoma has a luminal-papillary T-cell depleted contexture and activated FGFR3 signaling. Nat Commun. 2019; 10:2977.
Article23. Su X, Lu X, Bazai SK, Comperat E, Mouawad R, Yao H, et al. Comprehensive integrative profiling of upper tract urothelial carcinomas. Genome Biol. 2021; 22:7.
Article24. Kim K, Hu W, Audenet F, Almassi N, Hanrahan AJ, Murray K, et al. Modeling biological and genetic diversity in upper tract urothelial carcinoma with patient derived xenografts. Nat Commun. 2020; 11:1975.
Article25. Xylinas E, Kluth L, Passoni N, Trinh QD, Rieken M, Lee RK, et al. Prediction of intravesical recurrence after radical nephroureterectomy: development of a clinical decision-making tool. Eur Urol. 2014; 65:650–8.
Article26. Mao Y, Kilcoyne A, Hedgire S, Preston MA, McGovern FJ, Dahl DM, et al. Patterns of recurrence in upper tract transitional cell carcinoma: imaging surveillance. AJR Am J Roentgenol. 2016; 207:789–96.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Urothelial Tumors of the Upper Urinary Tract: Multiplicity and Prognostic Variables
- The Significance of Atypical Cell in Urinary Cytology
- Urothelial Tumors of the Upper Urinary Tract, 15 Cases
- A clinical analysis of the upper urinary tract transitional cell carcinoma and associated bladder tumor
- Nephron-sparing management of upper tract urothelial carcinoma